A structural model of the pore-forming region of the skeletal muscle ryanodine receptor (RyR1)
- PMID: 19390614
- PMCID: PMC2668181
- DOI: 10.1371/journal.pcbi.1000367
A structural model of the pore-forming region of the skeletal muscle ryanodine receptor (RyR1)
Abstract
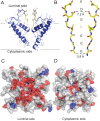
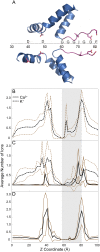
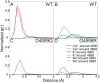
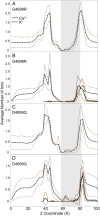
Ryanodine receptors (RyRs) are ion channels that regulate muscle contraction by releasing calcium ions from intracellular stores into the cytoplasm. Mutations in skeletal muscle RyR (RyR1) give rise to congenital diseases such as central core disease. The absence of high-resolution structures of RyR1 has limited our understanding of channel function and disease mechanisms at the molecular level. Here, we report a structural model of the pore-forming region of RyR1. Molecular dynamics simulations show high ion binding to putative pore residues D4899, E4900, D4938, and D4945, which are experimentally known to be critical for channel conductance and selectivity. We also observe preferential localization of Ca(2+) over K(+) in the selectivity filter of RyR1. Simulations of RyR1-D4899Q mutant show a loss of preference to Ca(2+) in the selectivity filter as seen experimentally. Electrophysiological experiments on a central core disease mutant, RyR1-G4898R, show constitutively open channels that conduct K(+) but not Ca(2+). Our simulations with G4898R likewise show a decrease in the preference of Ca(2+) over K(+) in the selectivity filter. Together, the computational and experimental results shed light on ion conductance and selectivity of RyR1 at an atomistic level.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. - PubMed
-
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev. 1997;77:699–729. - PubMed
-
- Dirksen RT, Avila G. Altered ryanodine receptor function in central core disease: leaky or uncoupled Ca2+ release channels? Trends Cardiovasc Med. 2002;12:189–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

