Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia
- PMID: 19390619
- PMCID: PMC2668697
- DOI: 10.1371/journal.ppat.1000395
Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia
Abstract
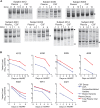
Human immunodeficiency virus type 1 (HIV-1) invades the central nervous system (CNS) shortly after systemic infection and can result in the subsequent development of HIV-1-associated dementia (HAD) in a subset of infected individuals. Genetically compartmentalized virus in the CNS is associated with HAD, suggesting autonomous viral replication as a factor in the disease process. We examined the source of compartmentalized HIV-1 in the CNS of subjects with HIV-1-associated neurological disease and in asymptomatic subjects who were initiating antiretroviral therapy. The heteroduplex tracking assay (HTA), targeting the variable regions of env, was used to determine which HIV-1 genetic variants in the cerebrospinal fluid (CSF) were compartmentalized and which variants were shared with the blood plasma. We then measured the viral decay kinetics of individual variants after the initiation of antiretroviral therapy. Compartmentalized HIV-1 variants in the CSF of asymptomatic subjects decayed rapidly after the initiation of antiretroviral therapy, with a mean half-life of 1.57 days. Rapid viral decay was also measured for CSF-compartmentalized variants in four HAD subjects (t(1/2) mean = 2.27 days). However, slow viral decay was measured for CSF-compartmentalized variants from an additional four subjects with neurological disease (t(1/2) range = 9.85 days to no initial decay). The slow decay detected for CSF-compartmentalized variants was not associated with poor CNS drug penetration, drug resistant virus in the CSF, or the presence of X4 virus genotypes. We found that the slow decay measured for CSF-compartmentalized variants in subjects with neurological disease was correlated with low peripheral CD4 cell count and reduced CSF pleocytosis. We propose a model in which infiltrating macrophages replace CD4(+) T cells as the primary source of productive viral replication in the CNS to maintain high viral loads in the CSF in a substantial subset of subjects with HAD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Boisse L, Gill MJ, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin. 2008;26:799–819. - PubMed
-
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. - PubMed
-
- Maslin CL, Kedzierska K, Webster NL, Muller WA, Crowe SM. Transendothelial migration of monocytes: the underlying molecular mechanisms and consequences of HIV-1 infection. Curr HIV Res. 2005;3:303–317. - PubMed
-
- Nottet HS, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, et al. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–1295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007001/AI/NIAID NIH HHS/United States
- R01 MH067751/MH/NIMH NIH HHS/United States
- T32-CA09156/CA/NCI NIH HHS/United States
- T32 CA009156/CA/NCI NIH HHS/United States
- R01 NS037660/NS/NINDS NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- K23-MH074466/MH/NIMH NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- R01-MH67751/MH/NIMH NIH HHS/United States
- UL1RR024131/RR/NCRR NIH HHS/United States
- P30-CA16086/CA/NCI NIH HHS/United States
- K23 MH074466/MH/NIMH NIH HHS/United States
- R01-NS37660/NS/NINDS NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- T32-AI07001/AI/NIAID NIH HHS/United States
- P30-AI50410/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

