Novel pathogenic mutations and skin biopsy analysis in Knobloch syndrome
- PMID: 19390655
- PMCID: PMC2671584
Novel pathogenic mutations and skin biopsy analysis in Knobloch syndrome
Abstract
Purpose: To facilitate future diagnosis of Knobloch syndrome (KS) and better understand its etiology, we sought to identify not yet described COL18A1 mutations in KS patients. In addition, we tested whether mutations in this gene lead to absence of the COL18A1 gene product and attempted to better characterize the functional effect of a previously reported missense mutation.
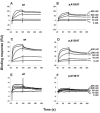
Methods: Direct sequencing of COL18A1 exons was performed in KS patients from four unrelated pedigrees. We used immunofluorescent histochemistry in skin biopsies to evaluate the presence of type XVIII collagen in four KS patients carrying two already described mutations: c.3277C>T, a nonsense mutation, and c.3601G>A, a missense mutation. Furthermore, we determined the binding properties of the mutated endostatin domain p.A1381T (c.3601G>A) to extracellular matrix proteins using ELISA and surface plasmon resonance assays.
Results: We identified four novel mutations in COL18A1, including a large deletion involving exon 41. Skin biopsies from KS patients revealed lack of type XVIII collagen in epithelial basement membranes and blood vessels. We also found a reduced affinity of p.A1381T endostatin to some extracellular matrix components.
Conclusions: COL18A1 mutations involved in Knobloch syndrome have a distribution bias toward the coding exons of the C-terminal end. Large deletions must also be considered when point mutations are not identified in patients with characteristic KS phenotype. We report, for the first time, lack of type XVIII collagen in KS patients by immunofluorescent histochemistry in skin biopsy samples. As a final point, we suggest the employment of this technique as a preliminary and complementary test for diagnosis of KS in cases when mutation screening either does not detect mutations or reveals mutations of uncertain effect, such as the p.A1381T change.
Figures


References
-
- Cohen MM, Lemire RJ. Syndromes with cephaloceles. Teratology. 1982;25:161–72. - PubMed
-
- Passos-Bueno MR, Marie SK, Monteiro M, Neustein I, Whittle MR, Vainzof M, Zatz M. Knobloch syndrome in a large Brazilian consanguineous family: confirmation of autosomal recessive inheritance. Am J Med Genet. 1994;52:170–3. - PubMed
-
- Sertié AL, Sossi V, Camargo AA, Zatz M, Brahe C, Passos-Bueno MR. Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retinal structure and in neural tube closure (Knobloch syndrome). Hum Mol Genet. 2000;9:2051–8. - PubMed
-
- Suzuki OT, Sertié AL, Der Kaloustian VM, Kok F, Carpenter M, Murray J, Czeizel AE, Kliemann SE, Rosemberg S, Monteiro M, Olsen BR, Passos-Bueno MR. Molecular analysis of collagen XVIII reveals novel mutations, presence of a third isoform, and possible genetic heterogeneity in Knobloch syndrome. Am J Hum Genet. 2002;71:1320–9. - PMC - PubMed
-
- Passos-Bueno MR, Suzuki OT, Armelin-Correa LM, Sertié AL, Errera FIV, Bagatini K, Kok F, Leite KRM. Mutations in collagen 18A1 and their relevance to the human phenotype. An Acad Bras Cienc. 2006;78:123–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
