Hedgehog signaling patterns mesoderm in the sea urchin
- PMID: 19393640
- PMCID: PMC2702090
- DOI: 10.1016/j.ydbio.2009.04.018
Hedgehog signaling patterns mesoderm in the sea urchin
Abstract
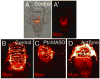
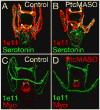
The Hedgehog (Hh) signaling pathway is essential for patterning many structures in vertebrates including the nervous system, chordamesoderm, limb and endodermal organs. In the sea urchin, a basal deuterostome, Hh signaling is shown to participate in organizing the mesoderm. At gastrulation the Hh ligand is expressed by the endoderm downstream of the Brachyury and FoxA transcription factors in the endomesoderm gene regulatory network. The co-receptors Patched (Ptc) and Smoothened (Smo) are expressed by the neighboring skeletogenic and non-skeletogenic mesoderm. Perturbations of Hh, Ptc and Smo cause embryos to develop with skeletal defects and inappropriate non-skeletogenic mesoderm patterning, although initial specification of mesoderm occurs without detectable abnormalities. Perturbations of the pathway caused late defects in skeletogenesis and in the non-skeletogenic mesoderm, including altered numbers of pigment and blastocoelar cells, randomized left-right asymmetry of coelomic pouches, and disorganized circumesophageal muscle causing an inability to swallow. Together the data support the requirement of Hh signaling in patterning each of the mesoderm subtypes in the sea urchin embryo.
Figures
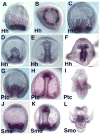
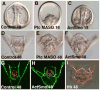

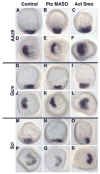

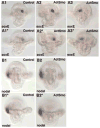


Similar articles
-
Contribution of hedgehog signaling to the establishment of left-right asymmetry in the sea urchin.Dev Biol. 2016 Mar 15;411(2):314-324. doi: 10.1016/j.ydbio.2016.02.008. Epub 2016 Feb 9. Dev Biol. 2016. PMID: 26872875 Free PMC article.
-
Genomics and expression profiles of the Hedgehog and Notch signaling pathways in sea urchin development.Dev Biol. 2006 Dec 1;300(1):153-64. doi: 10.1016/j.ydbio.2006.08.064. Epub 2006 Sep 1. Dev Biol. 2006. PMID: 17067570 Free PMC article.
-
LvGroucho and nuclear beta-catenin functionally compete for Tcf binding to influence activation of the endomesoderm gene regulatory network in the sea urchin embryo.Dev Biol. 2005 Mar 1;279(1):252-67. doi: 10.1016/j.ydbio.2004.12.023. Dev Biol. 2005. PMID: 15708573
-
Development gene networks and evolution.J Struct Funct Genomics. 2003;3(1-4):225-34. J Struct Funct Genomics. 2003. PMID: 12836701 Review.
-
Heads or tails? Amphioxus and the evolution of anterior-posterior patterning in deuterostomes.Dev Biol. 2002 Jan 15;241(2):209-28. doi: 10.1006/dbio.2001.0503. Dev Biol. 2002. PMID: 11784106 Review.
Cited by
-
Hedgehog Signaling in Intestinal Development and Homeostasis.Annu Rev Physiol. 2021 Feb 10;83:359-380. doi: 10.1146/annurev-physiol-031620-094324. Epub 2020 Oct 9. Annu Rev Physiol. 2021. PMID: 33035430 Free PMC article. Review.
-
Hedgehog signaling is required for endomesodermal patterning and germ cell development in the sea anemone Nematostella vectensis.Elife. 2020 Sep 24;9:e54573. doi: 10.7554/eLife.54573. Elife. 2020. PMID: 32969790 Free PMC article.
-
microRNA-1 regulates sea urchin skeletogenesis by directly targeting skeletogenic genes and modulating components of signaling pathways.Dev Biol. 2024 Apr;508:123-137. doi: 10.1016/j.ydbio.2024.01.010. Epub 2024 Jan 28. Dev Biol. 2024. PMID: 38290645 Free PMC article.
-
Notch and Nodal control forkhead factor expression in the specification of multipotent progenitors in sea urchin.Development. 2013 Apr;140(8):1796-806. doi: 10.1242/dev.091157. Development. 2013. PMID: 23533178 Free PMC article.
-
Evolutionary Changes in the Chromatin Landscape Contribute to Reorganization of a Developmental Gene Network During Rapid Life History Evolution in Sea Urchins.Mol Biol Evol. 2022 Sep 1;39(9):msac172. doi: 10.1093/molbev/msac172. Mol Biol Evol. 2022. PMID: 35946348 Free PMC article.
References
-
- Aihara M, Amemiya S. Left-right positioning of the adult rudiment in sea urchin larvae is directed by the right side. Development. 2001;128:4935–4948. - PubMed
-
- Armstrong N, Hardin J, McClay DR. Cell-cell interactions regulate skeleton formation in the sea urchin embryo. Development. 1993;119:833–840. - PubMed
-
- Armstrong N, McClay DR. Skeletal pattern is specified autonomously by the primary mesenchyme cells in sea urchin embryos. Dev Biol. 1994;162:329–338. - PubMed
-
- Aspock G, Kagoshima H, Niklaus G, Burglin TR. Caenorhabditis elegans has scores of hedgehog-related genes: sequence and expression analysis. Genome Res. 1999;9:909–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

