Protective actions of ovarian hormones in the serotonin system of macaques
- PMID: 19394356
- PMCID: PMC2704571
- DOI: 10.1016/j.yfrne.2009.04.003
Protective actions of ovarian hormones in the serotonin system of macaques
Abstract
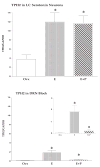
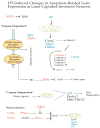
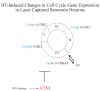
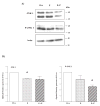
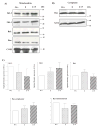
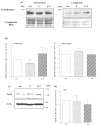
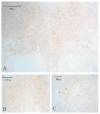
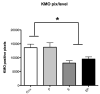
The serotonin neurons of the dorsal and medial raphe nuclei project to all areas of the forebrain and play a key role in mood disorders. Hence, any loss or degeneration of serotonin neurons could have profound ramifications. In a monkey model of surgical menopause with hormone replacement and no neural injury, E and P decreased gene expression in the dorsal raphe nucleus of c-jun n-terminal kinase (JNK1) and kynurenine mono-oxygenase (KMO) that promote cell death. In concert, E and P increased gene expression of superoxide dismutase (SOD1), VEGF, and caspase inhibitory proteins that promote cellular resilience in the dorsal raphe nucleus. Subsequently, we showed that ovarian steroids inhibit pivotal genes in the caspase-dependent and caspase-independent pathways in laser-captured serotonin neurons including apoptosis activating factor (Apaf1), apoptosis-inducing factor (AIF) and second mitochondria-derived activator of caspases (Smac/Diablo). SOD1 was also increased specifically in laser-captured serotonin neurons. Examination of protein expression in the dorsal raphe block revealed that JNK1, phosphoJNK1, AIF and the translocation of AIF from the mitochondria to the nucleus decreased with hormone therapy, whereas pivotal execution proteins in the caspase pathway were unchanged. In addition, cyclins A, B, D1 and E were inhibited, which would prevent re-entry into the cell cycle and catastrophic death. These data indicated that in the absence of gross injury to the midbrain, ovarian steroids inhibit the caspase-independent pathway and cell cycle initiation in serotonin neurons. To determine if these molecular actions prevented cellular vulnerability or death, we examined DNA fragmentation in the dorsal raphe nucleus with the TUNEL assay (terminal deoxynucleotidyl transferase nick end labeling). Ovarian steroids significantly decreased the number of TUNEL-positive cells in the dorsal raphe. Moreover, TUNEL staining prominently colocalized with TPH immunostaining, a marker for serotonin neurons. In summary, ovarian steroids increase the cellular resilience of serotonin neurons and may prevent serotonin neuron death in women facing decades of life after menopause. The survival of serotonin neurons would support cognition and mental health.
Figures












References
-
- Bethea CL. Colocalization of progestin receptors with serotonin in raphe neurons of macaque. Neuroendocrinology. 1993;57:1–6. - PubMed
-
- Gundlah C, Lu NZ, Mirkes SJ, Bethea CL. Estrogen receptor beta (ERβ) mRNA and protein in serotonin neurons of macaques. Molecular Brain Research. 2001;91:14–22. - PubMed
-
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res Mol Brain Res. 2005;135:194–203. - PubMed
-
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in Neuroendocrinology. 2002;23:41–100. - PubMed
-
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

