Enzymatic activity of alpha-L-fucosidase and L-fucokinase across vertebrate animal species
- PMID: 19394435
- PMCID: PMC3413248
- DOI: 10.1016/j.cbpb.2009.04.006
Enzymatic activity of alpha-L-fucosidase and L-fucokinase across vertebrate animal species
Abstract
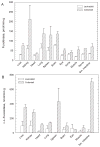
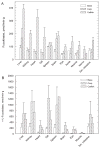
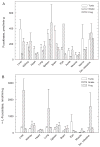
The oligosaccharide portion of glycoproteins is known to modulate protein structure, function, and turnover. Our laboratory is interested in the metabolism of L-fucose, a normal constituent of eukaryotic glycoproteins. L-fucose is unique in that it is the only levorotatory sugar utilized in mammalian systems. There is considerable interest in understanding the controls which determine the level of L-fucose attached to proteins, in order to generate stable and active glycoforms of protein for the treatment of disease. As part of a program to determine the controls on protein L-fucosylation, we have systematically determined the tissue distribution of the enzymes L-fucokinase and alpha-L-fucosidase in species across the vertebrate animal kingdom. In general, the level of alpha-L-fucosidase is higher than L-fucokinase level. The tissue with highest enzyme activity cannot be generalized, regardless of which enzyme is of interest. Furthermore, there is not a correlation between synthetic and catabolic enzyme activity within a tissue. L-fucokinase can be detected in all tissues examined. Interestingly, we have also detected ss-D-fucosidase activity, present in extraordinary levels in the liver and small intestine of snake. Whether this is due to a specific enzyme or whether it represents a broad specificity of the alpha-L-fucosidase is currently being investigated.
Figures


Similar articles
-
Comparative studies on the substrate specificity and defucosylation activity of three α-l-fucosidases using synthetic fucosylated glycopeptides and glycoproteins as substrates.Bioorg Med Chem. 2021 Jul 15;42:116243. doi: 10.1016/j.bmc.2021.116243. Epub 2021 Jun 7. Bioorg Med Chem. 2021. PMID: 34126284 Free PMC article.
-
Tissue distribution of L-fucokinase in rodents.Comp Biochem Physiol B Biochem Mol Biol. 2005 Mar;140(3):513-20. doi: 10.1016/j.cbpc.2004.11.018. Comp Biochem Physiol B Biochem Mol Biol. 2005. PMID: 15694600
-
Serum fucosylation changes in oral cancer and oral precancerous conditions: alpha-L-fucosidase as a marker.Cancer. 2008 Jul 15;113(2):336-46. doi: 10.1002/cncr.23556. Cancer. 2008. PMID: 18521898
-
Mammalian alpha-L-fucosidases.Comp Biochem Physiol B. 1991;99(3):479-88. doi: 10.1016/0305-0491(91)90327-a. Comp Biochem Physiol B. 1991. PMID: 1769200 Review.
-
[Purification and some properties of alpha-L-fucosidase isolated from Streptococcus sanguis ATCC 10557: role of the enzyme in degradation of salivary glycoprotein].Osaka Daigaku Shigaku Zasshi. 1978 Jun;23(1):1-16. Osaka Daigaku Shigaku Zasshi. 1978. PMID: 397322 Review. Japanese. No abstract available.
Cited by
-
Necrotizing Enterocolitis: The Role of Hypoxia, Gut Microbiome, and Microbial Metabolites.Int J Mol Sci. 2023 Jan 27;24(3):2471. doi: 10.3390/ijms24032471. Int J Mol Sci. 2023. PMID: 36768793 Free PMC article. Review.
References
-
- Angenstein F, Matthies H, Jr, Staeck S, Reymann KG, Staak S. The maintenance of hippocampal long-term potentiation is paralleled by a dopamine-dependent increase in glycoprotein fucosylation. Neurochem Int. 1992;21:403–408. - PubMed
-
- Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13:41R–53R. - PubMed
-
- Cabezas JA, Reglero A, Calvo P. Glycosidases (Fucosidases, galactosidases, glucosidases, hexosaminidases and glucuronidase from some molluscs and vertebrates, and neuraminidase from virus) [Review] Int J Biochem. 1983;15:243–259. - PubMed
-
- Fiete D, Mi Y, Oats EL, Beranek MC, Baenziger JU. N-Linked oligosaccharides on the low density lipoprotein receptor homolog SorLA/LR11 are modified with terminal GalNAc-4-SO4 in kidney and brain. J Biol Chem. 2007;282:1873–1881. - PubMed
-
- Flowers H. Chemistry and biochemistry of D- and L-fucose. Adv Carbohydrate Chem Biochem. 1981;39:279–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

