An exploratory dose-escalating study investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous atacicept in patients with systemic lupus erythematosus
- PMID: 19395457
- PMCID: PMC3835146
- DOI: 10.1177/0961203309102803
An exploratory dose-escalating study investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous atacicept in patients with systemic lupus erythematosus
Abstract
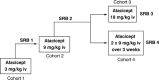
Atacicept, a recombinant fusion protein containing the extracellular, ligand-binding portion of the transmembrane activator and calcium modulator and cyclophilin-ligand interactor receptor, and the Fc portion of human immunoglobulin (Ig) G, is designed to block the activity of B-lymphocyte stimulator and a proliferation-inducing ligand, and may have utility as a treatment for B-cell-mediated diseases, such as systemic lupus erythematosus (SLE). This Phase Ib study investigated the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics of intravenous (i.v.) atacicept in patients with mild-to-moderate SLE. Patients (n = 24) were randomised (5:1) to receive atacicept (single dose: 3, 9 or 18 mg/kg; or multiple dose: 2 x 9 mg/kg) or matching placebo. Patients were followed for 6 weeks after dosing (9 weeks in the 2 x 9 mg/kg cohort). Local tolerability of atacicept was comparable with that of placebo, with only mild injection-site reactions reported with atacicept. Atacicept i.v. was generally well tolerated, both systemically and locally, in patients with mild-to-moderate SLE. Atacicept displayed non-linear PK, which was predictable across doses and between single and repeat doses. The biological activity of atacicept was demonstrated by its marked effect in reducing B-cells and Ig levels in patients with SLE. This supports the utility of this therapeutic approach in the treatment of autoimmune diseases, such as SLE.
Conflict of interest statement
CRP and HS are employees of Merck Serono S.A. (Geneva, Switzerland). At the time of the study, JH and IN were employees of ZymoGenetics Inc. (Seattle, WA, USA).
Figures



References
-
- Petri M. Clinical features of systemic lupus erythematosus. Curr Opin Rheumatol 1995; 7: 395–401 - PubMed
-
- Worrall JG, Snaith ML, Batchelor JR, Isenberg DA. SLE: A rheumatological view. Analysis of the clinical features, serology and immunogenetics of 100 SLE patients during long-term follow-up. Q J Med 1990; 74: 319–330 - PubMed
-
- Isenberg D, Rahman A. Systemic lupus erythematosus—2005 annus mirabilis? Nat Clin Pract Rheumatol 2006; 2: 145–152 - PubMed
-
- Moses N, Wiggers J, Nicholas C, Cockburn J. Prevalence and correlates of perceived unmet needs of people with systemic lupus erythematosus. Patient Educ Couns 2005; 57: 30–38 - PubMed
-
- Kyttaris VC, Katsiari CG, Juang YT, Tsokos GC. New insights into the pathogenesis of systemic lupus erythematosus. Curr Rheumatol Rep 2005; 7: 469–475 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

