beta-adrenergic regulation of a novel isoform of NCX: sequence and expression of shark heart NCX in human kidney cells
- PMID: 19395557
- PMCID: PMC2716097
- DOI: 10.1152/ajpheart.00038.2009
beta-adrenergic regulation of a novel isoform of NCX: sequence and expression of shark heart NCX in human kidney cells
Abstract
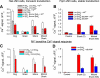
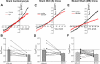
The function, regulation, and molecular structure of the cardiac Na(+)/Ca(2+) exchangers (NCXs) vary significantly among vertebrates. We previously reported that beta-adrenergic suppression of amphibian cardiac NCX1.1 is associated with specific molecular motifs. Here we investigated the bimodal, cAMP-dependent regulation of spiny dogfish shark (Squalus acanthias) cardiac NCX, exploring the effects of molecular structure, host cell environment, and ionic milieu. The shark cardiac NCX sequence (GenBank accession no. DQ 068478) revealed two novel proline/alanine-rich amino acid insertions. Wild-type and mutant shark NCXs were cloned and expressed in mammalian cells (HEK-293 and FlpIn-293), where their activities were measured as Ni(2+)-sensitive Ca(2+) fluxes (fluo 4) and membrane (Na(+)/Ca(2+) exchange) currents evoked by changes in extracellular Na(+) concentration and/or membrane potential. Regardless of Ca(2+) buffering, beta-adrenergic stimulation of cloned wild-type shark NCX consistently produced bimodal regulation (defined as differential regulation of Ca(2+)-efflux and -influx pathways), with suppression of the Ca(2+)-influx mode and either no change or enhancement of the Ca(2+)-efflux mode, closely resembling results from parallel experiments with native shark cardiomyocytes. In contrast, mutant shark NCX, with deletion of the novel region 2 insertion, produced equal suppression of the inward and outward currents and Ca(2+) fluxes, thereby abolishing the bimodal nature of the regulation. Control experiments with nontransfected and dog cardiac NCX-expressing cells showed no cAMP regulation. We conclude that bimodal beta-adrenergic regulation is retained in cloned shark NCX and is dependent on the shark's unique molecular motifs.
Figures




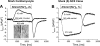


Similar articles
-
Three Na+/Ca2+ exchanger (NCX) variants are expressed in mouse osteoclasts and mediate calcium transport during bone resorption.Endocrinology. 2007 May;148(5):2116-25. doi: 10.1210/en.2006-1321. Epub 2007 Feb 22. Endocrinology. 2007. PMID: 17317768
-
Na+/Ca2+ exchanger overexpression impairs frequency- and ouabain-dependent cell shortening in adult rat cardiomyocytes.Am J Physiol Heart Circ Physiol. 2004 Oct;287(4):H1435-45. doi: 10.1152/ajpheart.00397.2003. Epub 2004 May 27. Am J Physiol Heart Circ Physiol. 2004. PMID: 15165985
-
Allosteric regulation of Na/Ca exchange current by cytosolic Ca in intact cardiac myocytes.J Gen Physiol. 2001 Feb;117(2):119-31. doi: 10.1085/jgp.117.2.119. J Gen Physiol. 2001. PMID: 11158165 Free PMC article.
-
Sodium-calcium exchangers (NCX): molecular hallmarks underlying the tissue-specific and systemic functions.Pflugers Arch. 2014 Jan;466(1):43-60. doi: 10.1007/s00424-013-1405-y. Epub 2013 Nov 27. Pflugers Arch. 2014. PMID: 24281864 Review.
-
Phosphorylation and other conundrums of Na/Ca exchanger, NCX1.Ann N Y Acad Sci. 2007 Mar;1099:103-18. doi: 10.1196/annals.1387.036. Ann N Y Acad Sci. 2007. PMID: 17446449 Review.
Cited by
-
Ca2+ signaling of human pluripotent stem cells-derived cardiomyocytes as compared to adult mammalian cardiomyocytes.Cell Calcium. 2020 Sep;90:102244. doi: 10.1016/j.ceca.2020.102244. Epub 2020 Jun 13. Cell Calcium. 2020. PMID: 32585508 Free PMC article. Review.
References
-
- Barry WH, Bridge JH. Intracellular calcium homeostasis in cardiac myocytes. Circulation 87: 1806–1815, 1993. - PubMed
-
- Bers DM, Bridge JH. Relaxation of rabbit ventricular muscle by Na-Ca exchange and sarcoplasmic reticulum calcium pump. Ryanodine and voltage sensitivity. Circ Res 65: 334–342, 1989. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

