Utilization of mucus from the coral Acropora palmata by the pathogen Serratia marcescens and by environmental and coral commensal bacteria
- PMID: 19395569
- PMCID: PMC2698349
- DOI: 10.1128/AEM.00457-09
Utilization of mucus from the coral Acropora palmata by the pathogen Serratia marcescens and by environmental and coral commensal bacteria
Abstract
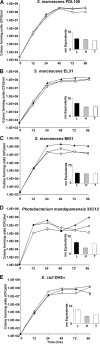
In recent years, diseases of corals caused by opportunistic pathogens have become widespread. How opportunistic pathogens establish on coral surfaces, interact with native microbiota, and cause disease is not yet clear. This study compared the utilization of coral mucus by coral-associated commensal bacteria ("Photobacterium mandapamensis" and Halomonas meridiana) and by opportunistic Serratia marcescens pathogens. S. marcescens PDL100 (a pathogen associated with white pox disease of Acroporid corals) grew to higher population densities on components of mucus from the host coral. In an in vitro coculture on mucus from Acropora palmata, S. marcescens PDL100 isolates outgrew coral isolates. The white pox pathogen did not differ from other bacteria in growth on mucus from a nonhost coral, Montastraea faveolata. The ability of S. marcescens to cause disease in acroporid corals may be due, at least in part, to the ability of strain PDL100 to build to higher population numbers within the mucus surface layer of its acroporid host. During growth on mucus from A. palmata, similar glycosidase activities were present in coral commensal bacteria, in S. marcescens PDL100, and in environmental and human isolates of S. marcescens. The temporal regulation of these activities during growth on mucus, however, was distinct in the isolates. During early stages of growth on mucus, enzymatic activities in S. marcescens PDL100 were most similar to those in coral commensals. After overnight incubation on mucus, enzymatic activities in a white pox pathogen were most similar to those in pathogenic Serratia strains isolated from human mucosal surfaces.
Figures




References
-
- Agresti, A. 1999. On logit confidence intervals for the odds ratio with small samples. Biometrics 55:597-602. - PubMed
-
- Brown, B. E., and J. C. Bythell. 2005. Perspectives on mucus secretion in reef corals. Mar. Ecol. Prog. Ser. 296:291-309.
-
- D'Aloia, M. A., T. A. Bailey, J. H. Samour, J. Naldo, and J. C. Howlett. 1996. Bacterial flora of captive houbara (Chlamydotis undulate), kori (Ardeotis kori) and rufous-crested (Eupodotis ruficrista) bustards. Avian Pathol. 3:459-468. - PubMed
-
- Ducklow, H. W., and R. Mitchell. 1979. Composition of mucus released by coral reef coelenterates. Limnol. Oceanogr. 21:706-714.
-
- Fabich, A. J., S. A. Jones, F. Z. Chowdhury, A. Cernosek, A. Anderson, D. Smalley, J. W. McHargue, G. A. Hightower, J. T. Smith, S. M. Autieri, M. P. Leatham, J. J. Lins, R. L. Allen, D. C. Laux, P. S. Cohen, and T. Conway. 2008. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect. Immun. 76:1143-1152. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

