Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart
- PMID: 19395641
- PMCID: PMC2673760
- DOI: 10.1242/dev.030924
Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart
Abstract
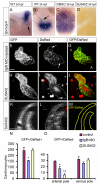
Amongst animal species, there is enormous variation in the size and complexity of the heart, ranging from the simple one-chambered heart of Ciona intestinalis to the complex four-chambered heart of lunged animals. To address possible mechanisms for the evolutionary adaptation of heart size, we studied how growth of the simple two-chambered heart in zebrafish is regulated. Our data show that the embryonic zebrafish heart tube grows by a substantial increase in cardiomyocyte number. Augmented cardiomyocyte differentiation, as opposed to proliferation, is responsible for the observed growth. By using transgenic assays to monitor developmental timing, we visualized for the first time the dynamics of cardiomyocyte differentiation in a vertebrate embryo. Our data identify two previously unrecognized phases of cardiomyocyte differentiation separated in time, space and regulation. During the initial phase, a continuous wave of cardiomyocyte differentiation begins in the ventricle, ends in the atrium, and requires Islet1 for its completion. In the later phase, new cardiomyocytes are added to the arterial pole, and this process requires Fgf signaling. Thus, two separate processes of cardiomyocyte differentiation independently regulate growth of the zebrafish heart. Together, our data support a model in which modified regulation of these distinct phases of cardiomyocyte differentiation has been responsible for the changes in heart size and morphology among vertebrate species.
Figures

References
-
- Berdougo, E., Coleman, H., Lee, D. H., Stainier, D. Y. and Yelon, D. (2003). Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development 130, 6121-6129. - PubMed
-
- Brade, T., Gessert, S., Kuhl, M. and Pandur, P. (2007). The amphibian second heart field: Xenopus islet-1 is required for cardiovascular development. Dev. Biol. 311, 297-310. - PubMed
-
- Buckingham, M. E., Meilhac, S. and Zaffran, S. (2005). Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 6, 826-835. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

