PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation
- PMID: 19396163
- PMCID: PMC2788612
- DOI: 10.1038/ncb1871
PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation
Abstract
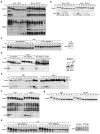
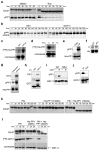
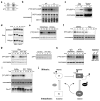
Loss of cell division cycle 2 (Cdc2, also known as Cdk1) activity after cyclin B degradation is necessary, but not sufficient, for mitotic exit. Proteins phosphorylated by Cdc2 and downstream mitotic kinases must be dephosphorylated. We report here that protein phosphatase-1 (PP1) is the main catalyst of mitotic phosphoprotein dephosphorylation. Suppression of PP1 during early mitosis is maintained through dual inhibition by Cdc2 phosphorylation and the binding of inhibitor-1. Protein kinase A (PKA) phosphorylates inhibitor-1, mediating binding to PP1. As Cdc2 levels drop after cyclin B degradation, auto-dephosphorylation of PP1 at its Cdc2 phosphorylation site (Thr 320) allows partial PP1 activation. This promotes PP1-regulated dephosphorylation at the activating site of inhibitor-1 (Thr 35) followed by dissociation of the inhibitor-1-PP1 complex and then full PP1 activation to promote mitotic exit. Thus, Cdc2 both phosphorylates multiple mitotic substrates and inhibits their PP1-mediated dephosphorylation.
Figures


References
-
- Lew DJ, Kornbluth S. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr Opin Cell Biol. 1996;8:795–804. - PubMed
-
- Azzam R, et al. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science. 2004;305:516–519. - PubMed
-
- Trautmann S, McCollum D. Cell cycle: new functions for Cdc14 family phosphatases. Curr Biol. 2002;12:R733–735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

