Green fluorescent proteins are light-induced electron donors
- PMID: 19396176
- PMCID: PMC2784199
- DOI: 10.1038/nchembio.174
Green fluorescent proteins are light-induced electron donors
Abstract
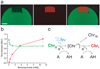
Proteins of the green fluorescent protein (GFP) family are well known owing to their unique biochemistry and extensive use as in vivo markers. We discovered that GFPs of diverse origins can act as light-induced electron donors in photochemical reactions with various electron acceptors, including biologically relevant ones. Moreover, via green-to-red GFP photoconversion, this process can be observed in living cells without additional treatment.
Figures

References
-
- Lippincott-Schwartz J, Patterson GH. Science. 2003;300:87–91. - PubMed
-
- Matz MV, et al. Nat. Biotechnol. 1999;17:969–973. - PubMed
-
- Shagin DA, et al. Mol. Biol. Evol. 2004;21:841–850. - PubMed
-
- Deheyn DD, et al. Biol. Bull. 2007;213:95–100. - PubMed
-
- Verkhusha VV, Lukyanov KA. Nat. Biotechnol. 2004;22:289–296. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

