Droplet interface bilayers
- PMID: 19396383
- PMCID: PMC2763081
- DOI: 10.1039/b808893d
Droplet interface bilayers
Abstract
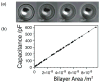
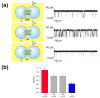
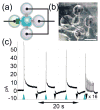
Droplet interface bilayers (DIBs) provide a superior platform for the biophysical analysis of membrane proteins. The versatile DIBs can also form networks, with features that include built-in batteries and sensors.
Figures










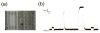



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

