Cobalt-induced oxidant stress in cultured endothelial cells: prevention by ascorbate in relation to HIF-1alpha
- PMID: 19396871
- PMCID: PMC2714551
- DOI: 10.1002/biof.43
Cobalt-induced oxidant stress in cultured endothelial cells: prevention by ascorbate in relation to HIF-1alpha
Abstract
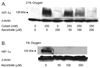
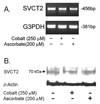
Endothelial cells respond to hypoxia by decreased degradation of hypoxia-inducible factor 1alpha (HIF-1alpha), accumulation of which leads to increased transcription of numerous proteins involved in cell growth and survival. Ascorbic acid prevents HIF-1alpha stabilization in many cell types, but the physiologic relevance of such effects is uncertain. Given their relevance for angiogenesis, endothelial cells in culture were used to evaluate the effects of ascorbate on HIF-1alpha expression induced by hypoxia and the hypoxia mimic cobalt. Although EA.hy926 cells in culture under oxygenated conditions did not contain ascorbate, HIF-1alpha expression was very low, showing that the vitamin is not necessary to suppress HIF-1alpha. On the other hand, hypoxia- or cobalt-induced HIF-1alpha expression/stabilization was almost completely suppressed by what are likely physiologic intracellular ascorbate concentrations. Increased HIF-1alpha expression was not associated with significant changes in expression of the SVCT2, the major transporter for ascorbate in these cells. Cobalt at concentrations sufficient to stabilize HIF-1alpha both oxidized intracellular ascorbate and induced an oxidant stress in the cells that was prevented by ascorbate. Whereas the interaction of ascorbate and cobalt is complex, the presence of physiologic low millimolar concentrations of ascorbate in endothelial cells effectively decreases HIF-1alpha expression and protects against cobalt-induced oxidant stress.
(c) 2009 International Union of Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Ascorbic acid, but not dehydroascorbic acid increases intracellular vitamin C content to decrease Hypoxia Inducible Factor -1 alpha activity and reduce malignant potential in human melanoma.Biomed Pharmacother. 2017 Feb;86:502-513. doi: 10.1016/j.biopha.2016.12.056. Epub 2016 Dec 23. Biomed Pharmacother. 2017. PMID: 28012930
-
Development of ascorbate transporters in brain cortical capillary endothelial cells in culture.Brain Res. 2008 May 7;1208:79-86. doi: 10.1016/j.brainres.2008.02.102. Epub 2008 Mar 18. Brain Res. 2008. PMID: 18394593 Free PMC article.
-
Oxidized lipoprotein induces the macrophage ascorbate transporter (SVCT2): protection by intracellular ascorbate against oxidant stress and apoptosis.Arch Biochem Biophys. 2009 May 15;485(2):174-82. doi: 10.1016/j.abb.2009.02.010. Epub 2009 Feb 28. Arch Biochem Biophys. 2009. PMID: 19254685 Free PMC article.
-
Ascorbate depletion mediates up-regulation of hypoxia-associated proteins by cell density and nickel.J Cell Biochem. 2006 Apr 1;97(5):1025-35. doi: 10.1002/jcb.20705. J Cell Biochem. 2006. PMID: 16288478
-
Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2.Free Radic Biol Med. 2009 Mar 15;46(6):719-30. doi: 10.1016/j.freeradbiomed.2008.12.018. Epub 2009 Jan 6. Free Radic Biol Med. 2009. PMID: 19162177 Free PMC article. Review.
Cited by
-
Exploring the Ascorbate Requirement of the 2-Oxoglutarate-Dependent Dioxygenases.J Med Chem. 2025 Feb 13;68(3):2219-2237. doi: 10.1021/acs.jmedchem.4c02342. Epub 2025 Jan 30. J Med Chem. 2025. PMID: 39883951 Free PMC article. Review.
-
Aged garlic extract and S-allylcysteine prevent apoptotic cell death in a chemical hypoxia model.Biol Res. 2016 Feb 1;49:7. doi: 10.1186/s40659-016-0067-6. Biol Res. 2016. PMID: 26830333 Free PMC article.
-
Melatonin counteracts cobalt nanoparticle‑induced cytotoxicity and genotoxicity by deactivating reactive oxygen species‑dependent mechanisms in the NRK cell line.Mol Med Rep. 2017 Oct;16(4):4413-4420. doi: 10.3892/mmr.2017.7309. Epub 2017 Aug 22. Mol Med Rep. 2017. PMID: 28849220 Free PMC article.
-
Sodium-dependent vitamin C transporter 2 (SVCT2) expression and activity in brain capillary endothelial cells after transient ischemia in mice.PLoS One. 2011 Feb 11;6(2):e17139. doi: 10.1371/journal.pone.0017139. PLoS One. 2011. PMID: 21347255 Free PMC article.
-
Increased Tumor Ascorbate is Associated with Extended Disease-Free Survival and Decreased Hypoxia-Inducible Factor-1 Activation in Human Colorectal Cancer.Front Oncol. 2014 Feb 4;4:10. doi: 10.3389/fonc.2014.00010. eCollection 2014. Front Oncol. 2014. PMID: 24551593 Free PMC article.
References
-
- Bruick RK, McKnight SL. Transcription. Oxygen sensing gets a second wind. Science. 2002;295:807–808. - PubMed
-
- Schofield CJ, Ratcliffe PJ. Signalling hypoxia by HIF hydroxylases. Biochem. Biophys. Res. Commun. 2005;338:617–626. - PubMed
-
- Semenza GL. Life with oxygen. Science. 2007;318:62–64. - PubMed
-
- Stolze IP, Mole DR, Ratcliffe PJ. Regulation of HIF: prolyl hydroxylases. Novartis. Found. Symp. 2006;272:15–25. - PubMed
-
- Hirsilä M, Koivunen P, Gunzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J. Biol. Chem. 2003;278:30772–30780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

