Altered senescence, apoptosis, and DNA damage response in a mutant p53 model of accelerated aging
- PMID: 19396980
- PMCID: PMC2722837
- DOI: 10.1016/j.mad.2009.01.001
Altered senescence, apoptosis, and DNA damage response in a mutant p53 model of accelerated aging
Abstract
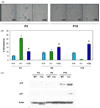
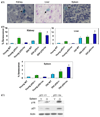
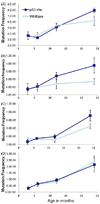
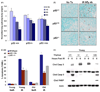
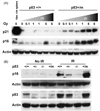
The tumor suppressors p16(INK4a) and p53 have been implicated as contributors to age-associated stem cell decline. Key functions of p53 are the induction of cell cycle arrest, senescence, or apoptosis in response to DNA damage. Here, we examine senescence, apoptosis, and DNA damage responses in a mouse accelerated aging model that exhibits increased p53 activity, the p53(+/m) mouse. Aged tissues of p53(+/m) mice display higher percentages of senescent cells (as determined by senescence-associated beta-galactosidase staining and p16(INK4a) and p21 accumulation) compared to aged tissues from p53(+/+) mice. Surprisingly, despite having enhanced p53 activity, p53(+/m) lymphoid tissues exhibit reduced apoptotic activity in response to ionizing radiation compared to p53(+/+) tissues. Ionizing radiation treatment of p53(+/m) tissues also induces higher and prolonged levels of senescence markers p16(INK4a) and p21, suggesting that in p53(+/m) tissues the p53 stress response is enhanced and is shifted away from apoptosis toward senescence. One potential mechanism for accelerated aging in the p53(+/m) mouse is a failure to remove damaged or dysfunctional cells (including stem and progenitor cells) through apoptosis. The increased accumulation of dysfunctional and senescent cells may contribute to reduced tissue regeneration, tissue atrophy, and some of the accelerated aging phenotypes in p53(+/m) mice.
Figures
Similar articles
-
p16(INK4a) protects against dysfunctional telomere-induced ATR-dependent DNA damage responses.J Clin Invest. 2013 Oct;123(10):4489-501. doi: 10.1172/JCI69574. Epub 2013 Sep 16. J Clin Invest. 2013. PMID: 24091330 Free PMC article.
-
Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice.Aging Cell. 2020 Mar;19(3):e13094. doi: 10.1111/acel.13094. Epub 2020 Jan 25. Aging Cell. 2020. PMID: 31981461 Free PMC article.
-
Single-cell analysis of p16(INK4a) and p21(WAF1) expression suggests distinct mechanisms of senescence in normal human and Li-Fraumeni Syndrome fibroblasts.J Cell Physiol. 2010 Apr;223(1):57-67. doi: 10.1002/jcp.22002. J Cell Physiol. 2010. PMID: 20039273
-
Two faces of p53: aging and tumor suppression.Nucleic Acids Res. 2007;35(22):7475-84. doi: 10.1093/nar/gkm744. Epub 2007 Oct 16. Nucleic Acids Res. 2007. PMID: 17942417 Free PMC article. Review.
-
Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells.Front Endocrinol (Lausanne). 2022 Mar 31;13:869414. doi: 10.3389/fendo.2022.869414. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432205 Free PMC article. Review.
Cited by
-
Postnatal development, maturation and aging in the mouse cochlea and their effects on hair cell regeneration.Hear Res. 2013 Mar;297:68-83. doi: 10.1016/j.heares.2012.11.009. Epub 2012 Nov 16. Hear Res. 2013. PMID: 23164734 Free PMC article. Review.
-
BRD4 Connects Enhancer Remodeling to Senescence Immune Surveillance.Cancer Discov. 2016 Jun;6(6):612-29. doi: 10.1158/2159-8290.CD-16-0217. Epub 2016 Apr 20. Cancer Discov. 2016. PMID: 27099234 Free PMC article.
-
Combined activation of the energy and cellular-defense pathways may explain the potent anti-senescence activity of methylene blue.Redox Biol. 2015 Dec;6:426-435. doi: 10.1016/j.redox.2015.09.004. Epub 2015 Sep 10. Redox Biol. 2015. PMID: 26386875 Free PMC article.
-
The cell cycle regulator p16 promotes tumor infiltrated CD8+ T cell exhaustion and apoptosis.Cell Death Dis. 2024 May 15;15(5):339. doi: 10.1038/s41419-024-06721-7. Cell Death Dis. 2024. PMID: 38750022 Free PMC article.
-
The quiescent cellular state is Arf/p53-dependent and associated with H2AX downregulation and genome stability.Int J Mol Sci. 2012;13(5):6492-6506. doi: 10.3390/ijms13056492. Epub 2012 May 24. Int J Mol Sci. 2012. PMID: 22754379 Free PMC article. Review.
References
-
- Adams CS, Horton WE., Jr Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat. Rec. 1998;250(4):418–425. - PubMed
-
- Bauer JH, Poon PC, Glatt-Deeley H, Abrams JM, Helfand SL. Neuronal expression of p53 dominant-negative proteins in adult Drosophila melanogaster extends life span. Curr. Biol. 2005;15(22):2063–2068. - PubMed
-
- Campisi J. Cancer and ageing: rival demons? Nat. Rev. Cancer. 2003;3(5):339–349. - PubMed
-
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

