Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal
- PMID: 19397281
- PMCID: PMC2685875
- DOI: 10.1021/tx9000489
Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal
Abstract
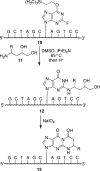
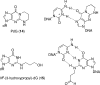
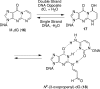
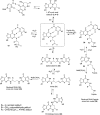
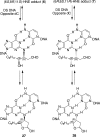
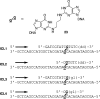
The alpha,beta-unsaturated aldehydes (enals) acrolein, crotonaldehyde, and trans-4-hydroxynonenal (4-HNE) are products of endogenous lipid peroxidation, arising as a consequence of oxidative stress. The addition of enals to dG involves Michael addition of the N(2)-amine to give N(2)-(3-oxopropyl)-dG adducts, followed by reversible cyclization of N1 with the aldehyde, yielding 1,N(2)-dG exocyclic products. The 1,N(2)-dG exocyclic adducts from acrolein, crotonaldehyde, and 4-HNE exist in human and rodent DNA. The enal-induced 1,N(2)-dG lesions are repaired by the nucleotide excision repair pathway in both Escherichia coli and mammalian cells. Oligodeoxynucleotides containing structurally defined 1,N(2)-dG adducts of acrolein, crotonaldehyde, and 4-HNE were synthesized via a postsynthetic modification strategy. Site-specific mutagenesis of enal adducts has been carried out in E. coli and various mammalian cells. In all cases, the predominant mutations observed are G-->T transversions, but these adducts are not strongly miscoding. When placed into duplex DNA opposite dC, the 1,N(2)-dG exocyclic lesions undergo ring opening to the corresponding N(2)-(3-oxopropyl)-dG derivatives. Significantly, this places a reactive aldehyde in the minor groove of DNA, and the adducted base possesses a modestly perturbed Watson-Crick face. Replication bypass studies in vitro indicate that DNA synthesis past the ring-opened lesions can be catalyzed by pol eta, pol iota, and pol kappa. It also can be accomplished by a combination of Rev1 and pol zeta acting sequentially. However, efficient nucleotide insertion opposite the 1,N(2)-dG ring-closed adducts can be carried out only by pol iota and Rev1, two DNA polymerases that do not rely on the Watson-Crick pairing to recognize the template base. The N(2)-(3-oxopropyl)-dG adducts can undergo further chemistry, forming interstrand DNA cross-links in the 5'-CpG-3' sequence, intrastrand DNA cross-links, or DNA-protein conjugates. NMR and mass spectrometric analyses indicate that the DNA interstand cross-links contain a mixture of carbinolamine and Schiff base, with the carbinolamine forms of the linkages predominating in duplex DNA. The reduced derivatives of the enal-mediated N(2)-dG:N(2)-dG interstrand cross-links can be processed in mammalian cells by a mechanism not requiring homologous recombination. Mutations are rarely generated during processing of these cross-links. In contrast, the reduced acrolein-mediated N(2)-dG peptide conjugates can be more mutagenic than the corresponding monoadduct. DNA polymerases of the DinB family, pol IV in E. coli and pol kappa in human, are implicated in error-free bypass of model acrolein-mediated N(2)-dG secondary adducts, the interstrand cross-links, and the peptide conjugates.
Figures
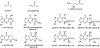
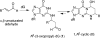
References
-
- Burcham P. C. (1998) Genotoxic lipid peroxidation products: Their DNA damaging properties and role in formation of endogenous DNA adducts. Mutagenesis 13, 287–305. - PubMed
-
- Chung F. L.; Zhang L.; Ocando J. E.; Nath R. G. (1999) Role of 1,N2-propanodeoxyguanosine adducts as endogenous DNA lesions in rodents and humans. IARC Sci. Publ. 150, 45–54. - PubMed
-
- Chung F. L.; Nath R. G.; Nagao M.; Nishikawa A.; Zhou G. D.; Randerath K. (1999) Endogenous formation and significance of 1,N2-propanodeoxyguanosine adducts. Mutat. Res. 424, 71–81. - PubMed
-
- Nair U.; Bartsch H.; Nair J. (2007) Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: A review of published adduct types and levels in humans. Free Radical Biol. Med. 43, 1109–1120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

