Replication past the N5-methyl-formamidopyrimidine lesion of deoxyguanosine by DNA polymerases and an improved procedure for sequence analysis of in vitro bypass products by mass spectrometry
- PMID: 19397282
- PMCID: PMC2754150
- DOI: 10.1021/tx900047c
Replication past the N5-methyl-formamidopyrimidine lesion of deoxyguanosine by DNA polymerases and an improved procedure for sequence analysis of in vitro bypass products by mass spectrometry
Abstract
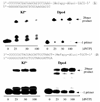
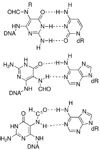
Oligonucleotides containing a site-specific N(6)-(2-deoxy-d-erythro-pentofuranosyl)-2,6-diamino-3,4-dihydro-4-oxo-5-N-methylformamidopyrimidine (MeFapy-dGuo) lesion were synthesized, and their in vitro replication by Escherichia coli DNA polymerase I Klenow fragment (exo(-)) and Sulfolobus solfataricus P2 DNA polymerase IV (Dpo4) resulted in the misincorporation of Ade, Gua, and Thy opposite the MeFapy-dGuo lesion in addition to the correct insertion of Cyt. However, sequencing of the full-length extension products revealed that the initial insertion of Cyt opposite the lesion was extended most efficiently. Two sequences were examined, and the misincorporation was sequence-dependent. Improvements in the method for the mass spectrometric sequencing of the extension products were developed; a 5'-biotinylated primer strand was used that contained a dUrd near the template-primer junction. The extended primer was immobilized with streptavidin-coated beads, allowing it to be washed free of polymerase, the template strand, and other reagents. The extended primer was cleaved from the solid support with uridine DNA deglycosylase and piperidine treatment, and the extension products were sequenced by LC-ESI-MS-MS. The purification steps afforded by the biotinylated primer resulted in improved sensitivity for the MS analysis. Translesion synthesis of a template with a local 5'-T-(MeFapy-dGuo)-G-3' sequence resulted in only error-free bypass and extension, whereas a template with a local 5'-T-(MeFapy-dGuo)-T-3' sequence also resulted in an interesting deletion product and the misincorporation of Ade opposite the MeFapy-dGuo lesion.
Figures





References
-
- Gates KS, Nooner T, Dutta S. Biologically relevant chemical reactions of N7-alkylguanine residues in DNA. Chem. Res. Toxicol. 2004;17:839–856. - PubMed
-
- Boiteux S, Guillet M. Abasic sites in DNA: Repair and biological consequences in Saccharomyces cerevisiae. DNA Repair. 2004;3:1–12. - PubMed
-
- Loeb LA, Preston DB. Mutagenesis by apurinic/apyrimidinic sites. Annu. Rev. Genet. 1986;20:201–230. - PubMed
-
- Greenberg MM. In vitro and in vivo effects of oxidative damage to deoxyguanosine. Biochem. Soc. Trans. 2004;32:46–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

