Replacement of a thiourea-S with an amidine-NH donor group in a platinum-acridine antitumor compound reduces the metal's reactivity with cysteine sulfur
- PMID: 19397321
- PMCID: PMC2693338
- DOI: 10.1021/jm900451y
Replacement of a thiourea-S with an amidine-NH donor group in a platinum-acridine antitumor compound reduces the metal's reactivity with cysteine sulfur
Abstract
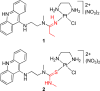
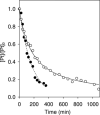
The reactivity of two DNA-targeted platinum-acridine conjugates with cysteine sulfur was studied. The conjugate containing an amidine-NH donor group cis to the chloride leaving group showed considerably reduced reactivity with N-acetylcysteine compared to the prototypical derivative containing a thiourea-S linkage. The opposite scenario has been observed previously in reactions with nucleobase nitrogen. Possible consequences of the unique target-selective tuning of the substitution chemistry for the pharmacodynamic properties and biological activity of these agents are discussed.
Figures
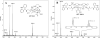
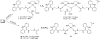
Similar articles
-
Replacement of a thiourea with an amidine group in a monofunctional platinum-acridine antitumor agent. Effect on DNA interactions, DNA adduct recognition and repair.Mol Pharm. 2011 Oct 3;8(5):1941-54. doi: 10.1021/mp200309x. Epub 2011 Aug 17. Mol Pharm. 2011. PMID: 21806015 Free PMC article.
-
Thermally inert metal ammines as light-inducible DNA-targeted agents. Synthesis, photochemistry, and photobiology of a prototypical rhodium(III)-intercalator conjugate.Inorg Chem. 2002 Dec 30;41(26):7159-69. doi: 10.1021/ic025744n. Inorg Chem. 2002. PMID: 12495358
-
Effect of linkage geometry on biological activity in thiourea- and guanidine-substituted acridines and platinum-acridines.Bioorg Med Chem Lett. 2008 Jul 1;18(13):3799-801. doi: 10.1016/j.bmcl.2008.05.043. Epub 2008 May 16. Bioorg Med Chem Lett. 2008. PMID: 18515101 Free PMC article.
-
DNA-binding antitumor agents: from pyrimido[5,6,1-de]acridines to other intriguing classes of acridine derivatives.Curr Med Chem. 2002 Sep;9(18):1701-16. doi: 10.2174/0929867023369268. Curr Med Chem. 2002. PMID: 12171552 Review.
-
A review on acridinylthioureas and its derivatives: biological and cytotoxic activity.J Appl Toxicol. 2017 Oct;37(10):1132-1139. doi: 10.1002/jat.3464. Epub 2017 Mar 31. J Appl Toxicol. 2017. PMID: 28370171 Review.
Cited by
-
Design and cellular studies of a carbon nanotube-based delivery system for a hybrid platinum-acridine anticancer agent.J Inorg Biochem. 2016 Dec;165:170-180. doi: 10.1016/j.jinorgbio.2016.07.016. Epub 2016 Jul 27. J Inorg Biochem. 2016. PMID: 27496614 Free PMC article.
-
Synthetic methods for the preparation of platinum anticancer complexes.Chem Rev. 2014 Apr 23;114(8):4470-95. doi: 10.1021/cr4004314. Epub 2013 Nov 27. Chem Rev. 2014. PMID: 24283498 Free PMC article. Review. No abstract available.
-
Development of Novel 191Pt-Labeled Hoechst33258: 191Pt Is More Suitable than 111In for Targeting DNA.J Med Chem. 2022 Apr 14;65(7):5690-5700. doi: 10.1021/acs.jmedchem.1c02209. Epub 2022 Mar 31. J Med Chem. 2022. PMID: 35358392 Free PMC article.
-
Design of enzymatically cleavable prodrugs of a potent platinum-containing anticancer agent.Chemistry. 2014 Dec 1;20(49):16164-73. doi: 10.1002/chem.201404675. Epub 2014 Oct 10. Chemistry. 2014. PMID: 25303639 Free PMC article.
-
Platination of cysteine by an epidermal growth factor receptor kinase-targeted hybrid agent.Chem Commun (Camb). 2018 Jul 11;54(54):7479-7482. doi: 10.1039/c8cc04251a. Epub 2018 Jun 19. Chem Commun (Camb). 2018. PMID: 29915817 Free PMC article.
References
-
- Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am. J. Med. Sci. 2007;334:115–124. - PubMed
-
- Wang X, Guo Z. The role of sulfur in platinum anticancer chemotherapy. Anticancer Agents Med. Chem. 2007;7:19–34. - PubMed
-
- Barnham KJ, Djuran MI, Murdoch PS, Ranford JD, Sadler PJ. Ring-Opened Adducts of the Anticancer Drug Carboplatin with Sulfur Amino Acids. Inorg. Chem. 1996;35:1065–1072. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

