Coexpression of human alpha- and circularly permuted beta-globins yields a hemoglobin with normal R state but modified T state properties
- PMID: 19397368
- PMCID: PMC2725443
- DOI: 10.1021/bi900216p
Coexpression of human alpha- and circularly permuted beta-globins yields a hemoglobin with normal R state but modified T state properties
Abstract
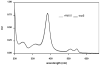
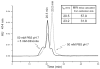
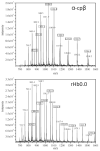
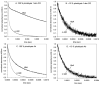
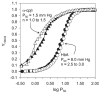
For the first time, a circularly permuted human beta-globin (cpbeta) has been coexpressed with human alpha-globin in bacterial cells and shown to associate to form alpha-cpbeta hemoglobin in solution. Flash photolysis studies of alpha-cpbeta show markedly biphasic CO and O(2) kinetics with the amplitudes for the fast association phases being dominant due the presence of large amounts of high-affinity liganded hemoglobin dimers. Extensive dimerization of liganded but not deoxygenated alpha-cpbeta was observed by gel chromatography. The rate constants for O(2) and CO binding to the R state forms of alpha-cpbeta are almost identical to those of native HbA (k'(R(CO)) approximately 5.0 microM(-1) s(-1); k'(R(O(2))) approximately 50 microM(-1) s(-1)), and the rate of O(2) dissociation from fully oxygenated alpha-cpbeta is also very similar to that observed for HbA (k(R(O(2))) approximately 21-28 s(-1)). When the equilibrium deoxyHb form of alpha-cpbeta is reacted with CO in rapid mixing experiments, the observed time courses are monophasic and the observed bimolecular association rate constant is approximately 1.0 microM(-1) s(-1), which is intermediate between the R state rate measured in partial photolysis experiments (approximately 5 microM(-1) s(-1)) and that observed for T state deoxyHbA (k'(T(CO)) approximately 0.1 to 0.2 microM(-1) s(-1)). Thus the deoxygenated permutated beta subunits generate an intermediate, higher affinity, deoxyHb quaternary state. This conclusion is supported by equilibrium oxygen binding measurements in which alpha-cpbeta exhibits a P(50) of approximately 1.5 mmHg and a low n-value (approximately 1.3) at pH 7, 20 degrees C, compared to 8.5 mmHg and n approximately 2.8 for native HbA under identical, dilute conditions.
Figures


Similar articles
-
Preparation and kinetic characterization of a series of betaW37 variants of human hemoglobin A: evidence for high-affinity T quaternary structures.Biochemistry. 1998 Mar 31;37(13):4325-35. doi: 10.1021/bi970866q. Biochemistry. 1998. PMID: 9521753
-
A kinetic description of dioxygen motion within alpha- and beta-subunits of human hemoglobin in the R-state: geminate and bimolecular stages of the oxygenation reaction.Biochemistry. 2004 Feb 17;43(6):1675-84. doi: 10.1021/bi034928q. Biochemistry. 2004. PMID: 14769045
-
Mutational effects at the subunit interfaces of human hemoglobin: evidence for a unique sensitivity of the T quaternary state to changes in the hinge region of the alpha 1 beta 2 interface.Biochemistry. 2001 Oct 16;40(41):12357-68. doi: 10.1021/bi010988p. Biochemistry. 2001. PMID: 11591155
-
Assignment of rate constants for O2 and CO binding to alpha and beta subunits within R- and T-state human hemoglobin.Methods Enzymol. 1994;232:363-86. doi: 10.1016/0076-6879(94)32055-1. Methods Enzymol. 1994. PMID: 8057869 Review. No abstract available.
-
Hb Bakersfield (HBA1: c.151_152insGGAGCC): The Insertion of Arg-His Between Codons 49 and 50 of the α1-Globin Chain Leads to Increased Oxygen Affinity.Hemoglobin. 2017 Jan;41(1):1-5. doi: 10.1080/03630269.2017.1302467. Hemoglobin. 2017. PMID: 28532286 Review.
Cited by
-
Monodisperse 130 kDa and 260 kDa Recombinant Human Hemoglobin Polymers as Scaffolds for Protein Engineering of Hemoglobin-Based Oxygen Carriers.J Funct Biomater. 2012 Jan 13;3(1):61-78. doi: 10.3390/jfb3010061. J Funct Biomater. 2012. PMID: 24956516 Free PMC article.
-
Purification of hemoglobin from red blood cells using tangential flow filtration and immobilized metal ion affinity chromatography.J Chromatogr B Analyt Technol Biomed Life Sci. 2011 Jan 15;879(2):131-8. doi: 10.1016/j.jchromb.2010.11.021. Epub 2010 Dec 17. J Chromatogr B Analyt Technol Biomed Life Sci. 2011. PMID: 21195679 Free PMC article.
References
-
- Whitaker BI, Sullivan M. The 2005 Nationwide Blood Collection and Utilization Survey Report. American Association of Blood Banks; 2005. Historical Perspectives; pp. 51–54.
-
- Stowell CP, Levin J, Spiess BD, Winslow RM. Progress in the development of RBC substitutes. Transfusion. 2001;41:287–299. - PubMed
-
- Winslow R, editor. Blood Substitutes. Elsevier; London: 2006.
-
- Dou Y, Maillett DH, Eich RF, Olson JS. Myoglobin as a model system for designing heme protein based blood substitutes. Biophys Chem. 2002;98:127–148. - PubMed
-
- Estep T, Bucci E, Farmer M, Greenburg G, Harrington J, Kim HW, Klein H, Mitchell P, Nemo G, Olsen K, Palmer A, Valeri CR, Winslow R. Basic science focus on blood substitutes: a summary of the NHLBI Division of Blood Diseases and Resources Working Group Workshop. Transfusion. 2008;48:776–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

