Coexpression of human alpha- and circularly permuted beta-globins yields a hemoglobin with normal R state but modified T state properties
- PMID: 19397368
- PMCID: PMC2725443
- DOI: 10.1021/bi900216p
Coexpression of human alpha- and circularly permuted beta-globins yields a hemoglobin with normal R state but modified T state properties
Abstract
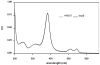
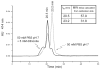
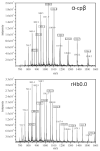
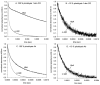
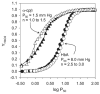
For the first time, a circularly permuted human beta-globin (cpbeta) has been coexpressed with human alpha-globin in bacterial cells and shown to associate to form alpha-cpbeta hemoglobin in solution. Flash photolysis studies of alpha-cpbeta show markedly biphasic CO and O(2) kinetics with the amplitudes for the fast association phases being dominant due the presence of large amounts of high-affinity liganded hemoglobin dimers. Extensive dimerization of liganded but not deoxygenated alpha-cpbeta was observed by gel chromatography. The rate constants for O(2) and CO binding to the R state forms of alpha-cpbeta are almost identical to those of native HbA (k'(R(CO)) approximately 5.0 microM(-1) s(-1); k'(R(O(2))) approximately 50 microM(-1) s(-1)), and the rate of O(2) dissociation from fully oxygenated alpha-cpbeta is also very similar to that observed for HbA (k(R(O(2))) approximately 21-28 s(-1)). When the equilibrium deoxyHb form of alpha-cpbeta is reacted with CO in rapid mixing experiments, the observed time courses are monophasic and the observed bimolecular association rate constant is approximately 1.0 microM(-1) s(-1), which is intermediate between the R state rate measured in partial photolysis experiments (approximately 5 microM(-1) s(-1)) and that observed for T state deoxyHbA (k'(T(CO)) approximately 0.1 to 0.2 microM(-1) s(-1)). Thus the deoxygenated permutated beta subunits generate an intermediate, higher affinity, deoxyHb quaternary state. This conclusion is supported by equilibrium oxygen binding measurements in which alpha-cpbeta exhibits a P(50) of approximately 1.5 mmHg and a low n-value (approximately 1.3) at pH 7, 20 degrees C, compared to 8.5 mmHg and n approximately 2.8 for native HbA under identical, dilute conditions.
Figures


References
-
- Whitaker BI, Sullivan M. The 2005 Nationwide Blood Collection and Utilization Survey Report. American Association of Blood Banks; 2005. Historical Perspectives; pp. 51–54.
-
- Stowell CP, Levin J, Spiess BD, Winslow RM. Progress in the development of RBC substitutes. Transfusion. 2001;41:287–299. - PubMed
-
- Winslow R, editor. Blood Substitutes. Elsevier; London: 2006.
-
- Dou Y, Maillett DH, Eich RF, Olson JS. Myoglobin as a model system for designing heme protein based blood substitutes. Biophys Chem. 2002;98:127–148. - PubMed
-
- Estep T, Bucci E, Farmer M, Greenburg G, Harrington J, Kim HW, Klein H, Mitchell P, Nemo G, Olsen K, Palmer A, Valeri CR, Winslow R. Basic science focus on blood substitutes: a summary of the NHLBI Division of Blood Diseases and Resources Working Group Workshop. Transfusion. 2008;48:776–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

