Laminin isoforms in human embryonic stem cells: synthesis, receptor usage and growth support
- PMID: 19397785
- PMCID: PMC6529980
- DOI: 10.1111/j.1582-4934.2008.00643.x
Laminin isoforms in human embryonic stem cells: synthesis, receptor usage and growth support
Abstract
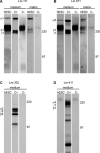
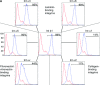
To reveal the functional intrinsic niche of human embryonic stem cells (hESC) we examined the production of basement membrane (BM) proteins and the presence of their receptors in feeder-free cell culture conditions. In addition, we investigated binding of hESCs to purified human BM proteins and identified the receptors mediating these contacts. Also, we tested whether purified human laminin (Lm) isoforms have a role in hESC self-renewal and growth in short-term cultures. The results show that hESCs synthesize Lm alpha(1) and Lm alpha(5) chains together with Lm beta(1) and gamma(1) chains suggesting the production of Lms-111 and -511 into the culture medium and deposits on cells. hESCs contain functionally important integrin (Int) subunits, Int beta(1), alpha(3), alpha(6), alpha(5), beta(5) and alpha(V), as well as the Lm alpha(5) receptor, Lutheran (Lu) glycoprotein and its truncated form, basal cell adhesion molecule (B-CAM). In cell adhesion experiments, Int beta(1) was crucial for adhesion to most of the purified human BM proteins. Lu/B-CAM mediated adhesion to Lm-511 together with Int alpha(3)beta(1), and was essential for the adhesion of hESCs to embryonic feeder cells. Adhesion to Lm-411 was mediated by Int alpha(6)beta(1). Lm-511 supported hESC growth in defined medium equally well as Matrigel. These results provide consequential information of the biological role of BM in hESCs, warranting further investigation of BM biology of human pluripotent stem cells.
Figures






Similar articles
-
Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells.Nat Commun. 2012;3:1236. doi: 10.1038/ncomms2231. Nat Commun. 2012. PMID: 23212365 Free PMC article.
-
Lutheran blood group antigen as a receptor for alpha5 laminins in gingival epithelia.J Periodontol. 2007 Sep;78(9):1810-8. doi: 10.1902/jop.2007.060482. J Periodontol. 2007. PMID: 17760553
-
Laminin isoform profiles in salivary glands in Sjögren's syndrome.Adv Clin Chem. 2011;55:35-59. doi: 10.1016/b978-0-12-387042-1.00003-4. Adv Clin Chem. 2011. PMID: 22126023 Review.
-
Blood vessels of human islets of Langerhans are surrounded by a double basement membrane.Diabetologia. 2008 Jul;51(7):1181-91. doi: 10.1007/s00125-008-0997-9. Epub 2008 Apr 26. Diabetologia. 2008. PMID: 18438639
-
Review: Lutheran/B-CAM: a laminin receptor on red blood cells and in various tissues.Connect Tissue Res. 2005;46(4-5):193-9. doi: 10.1080/03008200500344074. Connect Tissue Res. 2005. PMID: 16546822 Review.
Cited by
-
The role of laminins in cartilaginous tissues: from development to regeneration.Eur Cell Mater. 2017 Jul 21;34:40-54. doi: 10.22203/eCM.v034a03. Eur Cell Mater. 2017. PMID: 28731483 Free PMC article. Review.
-
Basement membrane components are key players in specialized extracellular matrices.Cell Mol Life Sci. 2010 Sep;67(17):2879-95. doi: 10.1007/s00018-010-0367-x. Epub 2010 Apr 29. Cell Mol Life Sci. 2010. PMID: 20428923 Free PMC article. Review.
-
The role of integrin β1 in the heterogeneity of human embryonic stem cells culture.Biol Open. 2018 Nov 1;7(11):bio034355. doi: 10.1242/bio.034355. Biol Open. 2018. PMID: 30385434 Free PMC article.
-
Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells.Nat Commun. 2012;3:1236. doi: 10.1038/ncomms2231. Nat Commun. 2012. PMID: 23212365 Free PMC article.
-
Comparison of several attachment methods for human iPS, embryonic and adipose-derived stem cells for tissue engineering.J Tissue Eng Regen Med. 2012 Dec;6 Suppl 3(0 3):s80-6. doi: 10.1002/term.1499. Epub 2012 May 18. J Tissue Eng Regen Med. 2012. PMID: 22610948 Free PMC article.
References
-
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer . 2003; 3: 422–33. - PubMed
-
- Scheele S, Nystrom A, Durbeej M, et al . Laminin isoforms in development and disease. J Mol Med . 2007; 85: 825–36. - PubMed
-
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Ann Rev Cell Dev Biol . 2004; 20: 255–84. - PubMed
-
- Ekblom P. Receptors for laminins during epithelial morphogenesis. Curr Opin Cell Biol . 1996; 8: 700–6. - PubMed
-
- Gustafsson E, Fassler R. Insights into extracellular matrix functions from mutant mouse models. Exp Cell Res . 2000; 261: 52–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

