The calcium-sensing receptor regulates parathyroid hormone gene expression in transfected HEK293 cells
- PMID: 19397786
- PMCID: PMC2681451
- DOI: 10.1186/1741-7007-7-17
The calcium-sensing receptor regulates parathyroid hormone gene expression in transfected HEK293 cells
Abstract
Background: The parathyroid calcium receptor determines parathyroid hormone secretion and the response of parathyroid hormone gene expression to serum Ca2+ in the parathyroid gland. Serum Ca2+ regulates parathyroid hormone gene expression in vivo post-transcriptionally affecting parathyroid hormone mRNA stability through the interaction of trans-acting proteins to a defined cis element in the parathyroid hormone mRNA 3'-untranslated region. These parathyroid hormone mRNA binding proteins include AUF1 which stabilizes and KSRP which destabilizes the parathyroid hormone mRNA. There is no parathyroid cell line; therefore, we developed a parathyroid engineered cell using expression vectors for the full-length human parathyroid hormone gene and the human calcium receptor.
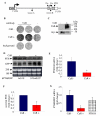
Results: Co-transfection of the human calcium receptor and the human parathyroid hormone plasmid into HEK293 cells decreased parathyroid hormone mRNA levels and secreted parathyroid hormone compared with cells that do not express the calcium receptor. The decreased parathyroid hormone mRNA correlated with decreased parathyroid hormone mRNA stability in vitro, which was dependent upon the 3'-UTR cis element. Moreover, parathyroid hormone gene expression was regulated by Ca2+ and the calcimimetic R568, in cells co-transfected with the calcium receptor but not in cells without the calcium receptor. RNA immunoprecipitation analysis in calcium receptor-transfected cells showed increased KSRP-parathyroid hormone mRNA binding and decreased binding to AUF1. The calcium receptor led to post-translational modifications in AUF1 as occurs in the parathyroid in vivo after activation of the calcium receptor.
Conclusion: The expression of the calcium receptor is sufficient to confer the regulation of parathyroid hormone gene expression to these heterologous cells. The calcium receptor decreases parathyroid hormone gene expression in these engineered cells through the parathyroid hormone mRNA 3'-UTR cis element and the balanced interactions of the trans-acting factors KSRP and AUF1 with parathyroid hormone mRNA, as in vivo in the parathyroid. This is the first demonstration that the calcium receptor can regulate parathyroid hormone gene expression in heterologous cells.
Figures




References
-
- Silver J, Naveh-Many T, Kronenberg HM. Parathyroid hormone: molecular biology. In: Bilezikian JB, Raisz LG, Rodan GA, editor. Principles of bone biology. San Diego: Academic Press; 2002. pp. 407–422.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

