Propofol: neuroprotection in an in vitro model of traumatic brain injury
- PMID: 19397790
- PMCID: PMC2689510
- DOI: 10.1186/cc7795
Propofol: neuroprotection in an in vitro model of traumatic brain injury
Abstract
Introduction: The anaesthetic agent propofol (2,6-diisopropylphenol) has been shown to be an effective neuroprotective agent in different in vitro models of brain injury induced by oxygen and glucose deprivation. We examined its neuroprotective properties in an in vitro model of traumatic brain injury.
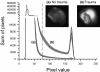
Methods: In this controlled laboratory study organotypic hippocampal brain-slice cultures were gained from six- to eight-day-old mice pups. After 14 days in culture, hippocampal brain slices were subjected to a focal mechanical trauma and subsequently treated with different molar concentrations of propofol under both normo- and hypothermic conditions. After 72 hours of incubation, tissue injury assessment was performed using propidium iodide (PI), a staining agent that becomes fluorescent only when it enters damaged cells via perforated cell membranes. Inside the cell, PI forms a fluorescent complex with nuclear DNA.
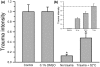
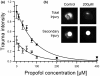
Results: A dose-dependent reduction of both total and secondary tissue injury could be observed in the presence of propofol under both normo- and hypothermic conditions. This effect was further amplified when the slices were incubated at 32 degrees C after trauma.
Conclusions: When used in combination, the dose-dependent neuroprotective effect of propofol is additive to the neuroprotective effect of hypothermia in an in vitro model of traumatic brain injury.
Figures


Similar articles
-
Argon: neuroprotection in in vitro models of cerebral ischemia and traumatic brain injury.Crit Care. 2009;13(6):R206. doi: 10.1186/cc8214. Epub 2009 Dec 17. Crit Care. 2009. PMID: 20017934 Free PMC article.
-
Neuroprotective properties of levosimendan in an in vitro model of traumatic brain injury.BMC Neurol. 2010 Oct 21;10:97. doi: 10.1186/1471-2377-10-97. BMC Neurol. 2010. PMID: 20964834 Free PMC article.
-
[The influences of propofol on different kinds of brain injuries in rat brain slices].Zhonghua Yi Xue Za Zhi. 2003 Jul 10;83(13):1176-9. Zhonghua Yi Xue Za Zhi. 2003. PMID: 12921640 Chinese.
-
Propofol: a review of its non-anaesthetic effects.Eur J Pharmacol. 2009 Mar 1;605(1-3):1-8. doi: 10.1016/j.ejphar.2009.01.007. Eur J Pharmacol. 2009. PMID: 19248246 Review.
-
Traumatic brain injury: neuroprotective anaesthetic techniques, an update.Injury. 2009 Nov;40 Suppl 4:S75-81. doi: 10.1016/j.injury.2009.10.040. Injury. 2009. PMID: 19895957 Review.
Cited by
-
Dexmedetomidine attenuates repeated propofol exposure-induced hippocampal apoptosis, PI3K/Akt/Gsk-3β signaling disruption, and juvenile cognitive deficits in neonatal rats.Mol Med Rep. 2016 Jul;14(1):769-75. doi: 10.3892/mmr.2016.5321. Epub 2016 May 23. Mol Med Rep. 2016. PMID: 27222147 Free PMC article.
-
Anesthetic propofol attenuates the isoflurane-induced caspase-3 activation and Aβ oligomerization.PLoS One. 2011;6(11):e27019. doi: 10.1371/journal.pone.0027019. Epub 2011 Nov 1. PLoS One. 2011. PMID: 22069482 Free PMC article.
-
Argon attenuates the emergence of secondary injury after traumatic brain injury within a 2-hour incubation period compared to desflurane: an in vitro study.Med Gas Res. 2017 Jun 30;7(2):93-100. doi: 10.4103/2045-9912.208512. eCollection 2017 Apr-Jun. Med Gas Res. 2017. PMID: 28744361 Free PMC article.
-
Therapeutic hypothermia as a neuroprotective strategy in neonatal hypoxic-ischemic brain injury and traumatic brain injury.Curr Mol Med. 2012 Dec;12(10):1282-96. doi: 10.2174/156652412803833517. Curr Mol Med. 2012. PMID: 22834830 Free PMC article. Review.
-
Propofol Protects Against Erastin-Induced Ferroptosis in HT-22 Cells.J Mol Neurosci. 2022 Sep;72(9):1797-1808. doi: 10.1007/s12031-022-02017-7. Epub 2022 Jun 21. J Mol Neurosci. 2022. PMID: 35727524
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

