Recruitment to a physical activity intervention study in women at increased risk of breast cancer
- PMID: 19397816
- PMCID: PMC2681472
- DOI: 10.1186/1471-2288-9-27
Recruitment to a physical activity intervention study in women at increased risk of breast cancer
Abstract
Background: Physical activity is being studied as a breast cancer prevention strategy. Women at risk of breast cancer report interest in lifestyle modification, but recruitment to randomized physical activity intervention studies is challenging.
Methods: We conducted an analysis of recruitment techniques used for a prospective, randomized pilot study of physical activity in women at risk of breast cancer. We evaluated differences in proportion of eligible patients, enrolled patients, and successful patients identified by each individual recruitment method. The Fisher-Freeman-Halton test (an extension of Fisher's exact test from 2 x 2 tables to general row by column tables) was used to compare the success of different recruitment strategies.
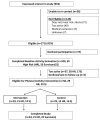
Results: We received 352 inquiries from women interested in participating, of whom 171 (54%) were eligible. Ninety-nine women completed a baseline activity evaluation, and 58 (34% of eligible; 16% of total inquiries) were randomized. Recruitment methods fell into three broad categories: media techniques, direct contact with potential participants, and contacts with health care providers. Recruitment strategies differed significantly in their ability to identify eligible women (p = 0.01), and women who subsequently enrolled in the study (p = 0.02).
Conclusion: Recruitment techniques had varying success. Our data illustrate the challenges in recruiting to behavior modification studies, and provide useful information for tailoring future recruitment efforts for lifestyle intervention trials. TRIAL REGISTRATION NO(S): CDR0000393790, NCI-04-C-0276, NCI-NAVY-B05-001.
Figures
References
-
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. Jama. 2006;295(23):2727–41. doi: 10.1001/jama.295.23.joc60074. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical