(1R, 3S)-(-)-trans-PAT: a novel full-efficacy serotonin 5-HT2C receptor agonist with 5-HT2A and 5-HT2B receptor inverse agonist/antagonist activity
- PMID: 19397907
- PMCID: PMC2749987
- DOI: 10.1016/j.ejphar.2009.04.035
(1R, 3S)-(-)-trans-PAT: a novel full-efficacy serotonin 5-HT2C receptor agonist with 5-HT2A and 5-HT2B receptor inverse agonist/antagonist activity
Abstract
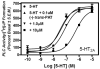
The serotonin 5-HT(2A), 5-HT(2B), and 5-HT(2C) G protein-coupled receptors signal primarily through G alpha(q) to activate phospholipase C (PLC) and formation of inositol phosphates (IP) and diacylglycerol. The human 5-HT(2C) receptor, expressed exclusively in the central nervous system, is involved in several physiological and psychological processes. Development of 5-HT(2C) agonists that do not also activate 5-HT(2A) or 5-HT(2B) receptors is challenging because transmembrane domain identity is about 75% among 5-HT(2) subtypes. This paper reports 5-HT(2) receptor affinity and function of (1R,3S)-(-)-trans-1-phenyl-3-dimethylamino-1,2,3,4-tetrahydronaphthalene (PAT), a small molecule that produces anorexia and weight-loss after peripheral administration to mice. (-)-Trans-PAT is a stereoselective full-efficacy agonist at human 5-HT(2C) receptors, plus, it is a 5-HT(2A)/5-HT(2B) inverse agonist and competitive antagonist. The K(i) of (-)-trans-PAT at 5-HT(2A), 5-HT(2B), and 5-HT(2C) receptors is 410, 1200, and 37 nM, respectively. Functional studies measured activation of PLC/[(3)H]-IP formation in clonal cells expressing human 5-HT(2) receptors. At 5-HT(2C) receptors, (-)-trans-PAT is an agonist (EC(50) = 20 nM) comparable to serotonin in potency and efficacy. At 5-HT(2A) and 5-HT(2B) receptors, (-)-trans-PAT is an inverse agonist (IC(50) = 490 and 1,000 nM, respectively) and competitive antagonist (K(B) = 460 and 1400 nM, respectively) of serotonin. Experimental results are interpreted in light of molecular modeling studies indicating the (-)-trans-PAT protonated amine can form an ionic bond with D3.32 of 5-HT(2A) and 5-HT(2C) receptors, but, not with 5-HT(2B) receptors. In addition to probing 5-HT(2) receptor structure and function, (-)-trans-PAT is a novel lead regarding 5-HT(2C) agonist/5-HT(2A) inverse agonist drug development for obesity and neuropsychiatric disorders.
Figures


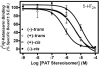
(A) [3H]-Ketanserin labeled 5-HT2A receptors; Bmax = 1.73 ± 0.11, Kd = 0.80 ± 0.03.
(B) [3H]-Mesulergine labeled 5-HT2B receptors; Bmax = 1.13 ± 0.39, Kd = 5.19 ± 0.36.
(C) [3H]-Mesulergine labeled 5-HT2C receptors; Bmax = 8.37 ± 0.15, Kd = 0.88 ± 0.03.





References
-
- Bakker RA, Dees G, Carrillo JJ, Booth RG, Lopez-Gimenez JF, Milligan G, Strange PG, Leurs R. Domain swapping in the human histamine H1 receptor. J Pharmacol Exp Ther. 2004;311:131–138. - PubMed
-
- Ballesteros JA, Shi L, Javitch JA. Structural mimicry in G protein-coupled receptors: implications of the high-resolution structure of rhodopsin for structure-function analysis of rhodopsin-like receptors. Mol Pharmacol. 2001;60:1–19. - PubMed
-
- Berendsen HJC, Postma JPM, van Gunsteren WF, Hermans J. Intermolecular Forces. D. Reidel Publishing Company; Dordrecht, the Netherlands: 1981. Interaction models for water in relation to protein hydration; pp. 331–342.
-
- Bivens NM, Weisstaub NV, Bradley-Moore M, Kevin K, Booth RG, Gingrich JA. 5HT2A and 5HT2C receptors in mouse models of psychosis and obesity Impact of a novel compound. American College of Neuropsychopharmacology Abstracts Annual Meeting; Boca Raton, Florida. December 9-13.2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

