The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study
- PMID: 19398283
- PMCID: PMC2846510
- DOI: 10.1016/j.drugalcdep.2009.02.014
The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study
Abstract
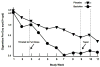
The surge in dopamine in ventral striatal regions in response to drugs of abuse and drug-associated stimuli is a final common pathway of addiction processes. GABA B agonists exert their effects indirectly, by quieting dopaminergic afferents. The ability of the GABA B agonist, baclofen to ameliorate nicotine and drug motivated behavior is established within the animal literature, however its potential to do so in humans is understudied, particularly with respect to its possible utility as a smoking cessation agent. We conducted a nine-week double-blind placebo-controlled pilot trial of baclofen for smoking reduction (N=30/group) in smokers contemplating, but not quite ready to quit. Baclofen was titrated upwards to 20mg q.i.d. over a period of twelve days. The primary outcome measure was the number of cigarettes smoked per day (CPD). A significant group by time effect of medication was observed. Baclofen was superior to placebo in reducing CPD (beta=0.01, t=1.97, p<0.05). The most common side effect reported during baclofen treatment is transient drowsiness, however there were no differences between groups in mild, moderate, or severe sedation. Craving was significantly lowered at end of treatment in all smokers (p<0.02). Retention did not differ between groups. In line with a multitude of preclinical studies examining the effects of baclofen on drug-motivated behavior, baclofen reduced CPD. In agreement with other studies examining craving and drug use, reductions in CPD were accompanied by a reduction in craving, a major motivator underlying continued smoking and relapse. These preliminary results demonstrate provisional evidence of the utility of baclofen to aid in smoking cessation and indicate further investigation.
Conflict of interest statement
None declared
Figures

References
-
- Addolorato G, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G. Ability of baclofen in reducing alcohol craving and intake: II--Preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24:67–71. - PubMed
-
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, Agabio R, Colombo G, Gessa GL, Gasbarrini G. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–508. - PubMed
-
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D’Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–1922. - PubMed
-
- Agabio R, Marras P, Addolorato G, Carpiniello B, Gessa GL. Baclofen suppresses alcohol intake and craving for alcohol in a schizophrenic alcohol-dependent patient: a case report. J Clin Psychopharmacol. 2007;27:319–320. - PubMed
-
- Aisen ML, Dietz MA, Rossi P, Cedarbaum JM, Kutt H. Clinical and pharmacokinetic aspects of high dose oral baclofen therapy. J Am Paraplegia Soc. 1992;15:211–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

