Nonhematopoietic cells control the outcome of infection with Listeria monocytogenes in a nucleotide oligomerization domain 1-dependent manner
- PMID: 19398545
- PMCID: PMC2708577
- DOI: 10.1128/IAI.01068-08
Nonhematopoietic cells control the outcome of infection with Listeria monocytogenes in a nucleotide oligomerization domain 1-dependent manner
Abstract
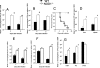
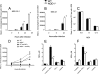
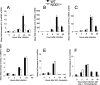
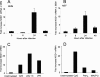
We analyzed the defensive role of the cytosolic innate recognition receptor nucleotide oligomerization domain 1 (NOD1) during infection with Listeria monocytogenes. Mice lacking NOD1 showed increased susceptibility to systemic intraperitoneal and intravenous infection with high or low doses of L. monocytogenes, as measured by the bacterial load and survival. NOD1 also controlled dissemination of L. monocytogenes into the brain. The increased susceptibility to reinfection of NOD1(-/-) mice was not associated with impaired triggering of listeria-specific T cells, and similar levels of costimulatory molecules or activation of dendritic cells was observed. Higher numbers of F480(+) Gr1(+) inflammatory monocytes and lower numbers of F480(-) Gr1(+) neutrophils were recruited into the peritoneum of infected WT mice than into the peritoneum of infected NOD1(-/-) mice. We determined that nonhematopoietic cells accounted for NOD1-mediated resistance to L. monocytogenes in bone marrow radiation chimeras. The levels of NOD1 mRNA in fibroblasts and bone marrow-derived macrophages (BMM) were upregulated after infection with L. monocytogenes or stimulation with different Toll-like receptor ligands. NOD1(-/-) BMM, astrocytes, and fibroblasts all showed enhanced intracellular growth of L monocytogenes compared to WT controls. Gamma interferon-mediated nitric oxide production and inhibition of L. monocytogenes growth were hampered in NOD1(-/-) BMM. Thus, NOD1 confers nonhematopoietic cell-mediated resistance to infection with L. monocytogenes and controls intracellular bacterial growth in different cell populations in vitro.
Figures
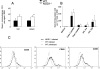
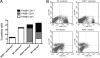
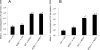
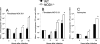
References
-
- Beckerman, K. P., H. W. Rogers, J. A. Corbett, R. D. Schreiber, M. L. McDaniel, and E. R. Unanue. 1993. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J. Immunol. 150888-895. - PubMed
-
- Carneiro, L. A., L. H. Travassos, and D. J. Philpott. 2004. Innate immune recognition of microbes through Nod1 and Nod2: implications for disease. Microbes Infect. 6609-616. - PubMed
-
- Chamaillard, M., S. E. Girardin, J. Viala, and D. J. Philpott. 2003. Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell. Microbiol. 5581-592. - PubMed
-
- Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4702-707. - PubMed
-
- Chin, A. I., P. W. Dempsey, K. Bruhn, J. F. Miller, Y. Xu, and G. Cheng. 2002. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature 416190-194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

