Targeted knock-in mice expressing mutations of CD28 reveal an essential pathway for costimulation
- PMID: 19398586
- PMCID: PMC2698768
- DOI: 10.1128/MCB.01869-08
Targeted knock-in mice expressing mutations of CD28 reveal an essential pathway for costimulation
Abstract
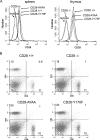
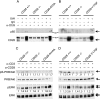
Despite extensive study, the role of phosphatidylinositol 3-kinase (PI3-kinase) activation in CD28 function has been highly contentious. To definitively address this question, we generated knock-in mice expressing mutations in two critical domains of the cytoplasmic tail of CD28. Mutation of the proximal tyrosine motif interrupted PI3-kinase binding and prevented CD28-dependent phosphorylation of protein kinase B (PKB)/Akt; however, there was no detectable effect on interleukin-2 (IL-2) secretion, expression of Bcl-X(L), or on T-cell function in vivo. Furthermore, we demonstrate that signaling initiated by the C-terminal proline motif is directly responsible for tyrosine phosphorylation of phosphoinosotide-dependent kinase 1, protein kinase C theta, and glycogen synthase kinase 3beta, as well as contributing to threonine phosphorylation of PKB. T cells mutated in this domain were profoundly impaired in IL-2 secretion, and the mice had marked impairment of humoral responses as well as less severe disease manifestations in experimental allergic encephalomyelitis. These data demonstrate that the distal proline motif initiates a critical nonredundant signaling pathway, whereas direct activation of PI3-kinase by the proximal tyrosine motif of CD28 is not required for normal T-cell function.
Figures
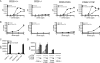
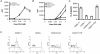




References
-
- Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7261-269. - PubMed
-
- Ali, A., K. P. Hoeflich, and J. R. Woodgett. 2001. Glycogen synthase kinase-3: properties, functions, and regulation. Chem. Rev. 1012527-2540. - PubMed
-
- Andres, P. G., K. C. Howland, A. Nirula, L. P. Kane, L. Barron, D. Dresnek, A. Sadra, J. Imboden, A. Weiss, and A. K. Abbas. 2004. Distinct regions in the CD28 cytoplasmic domain are required for T helper type 2 differentiation. Nat. Immunol. 5435-442. - PubMed
-
- Appleman, L. J., A. A. van Puijenbroek, K. M. Shu, L. M. Nadler, and V. A. Boussiotis. 2002. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J. Immunol. 1682729-2736. - PubMed
-
- Ballou, L. M., P.-Y. Tian, H.-Y. Lin, Y.-P. Jiang, and R. Z. Lin. 2001. Dual regulation of glycogen synthase kinase-3β by the α1A-adrenergic receptor. J. Biol. Chem. 27640910-40916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
