Participation of the urokinase receptor in neutrophil efferocytosis
- PMID: 19398720
- PMCID: PMC2716023
- DOI: 10.1182/blood-2008-12-193524
Participation of the urokinase receptor in neutrophil efferocytosis
Abstract
The urokinase receptor (uPAR) plays an important role in regulation of fibronolysis, cell migration, and adhesion. In this study, we examined whether uPAR plays a role in modulating efferocytosis of neutrophils. Macrophages from uPAR(-/-) mice demonstrated enhanced ability to engulf viable wild-type (WT) neutrophils in vitro and in vivo in the lungs. The increased phagocytic activity of uPAR(-/-) macrophages was abrogated by incubation with soluble uPAR (suPAR), arginine-glycine-aspartic acid (RGD)-containing peptides, or anti-integrin antibodies. There was increased uptake of viable uPAR(-/-) neutrophils by WT macrophages. Incubation of uPAR(-/-) neutrophils with suPAR or anti-integrin antibodies diminished uptake by WT macrophages to baseline. Uptake of uPAR(-/-) neutrophils by uPAR(-/-) macrophages was not enhanced. However, incubation of uPAR(-/-) neutrophils or uPAR(-/-) macrophages, but not both, with suPAR enhanced the uptake of viable uPAR(-/-) neutrophils by uPAR(-/-) macrophages. The adhesion of WT neutrophils to uPAR(-/-) macrophages was higher than to WT macrophages. uPAR(-/-) neutrophils demonstrated increased adhesion to suPAR, which was abrogated by blocking of low-density lipoprotein related protein and integrins. Expression of uPAR on the surface of apoptotic neutrophils was reduced compared with levels on viable neutrophils. These results demonstrate a novel role for uPAR in modulating recognition and clearance of neutrophils.
Figures
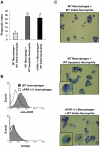
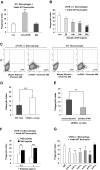
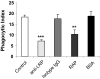
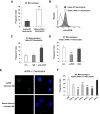
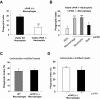
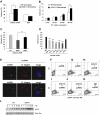
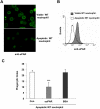
Comment in
-
Efferocytosis: another function of uPAR.Blood. 2009 Jul 23;114(4):752-3. doi: 10.1182/blood-2009-05-220657. Blood. 2009. PMID: 19628714
References
-
- Henson PM, Tuder RM. Apoptosis in the lung: induction, clearance and detection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L601–L611. - PubMed
-
- Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. - PubMed
-
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

