M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis
- PMID: 19398755
- PMCID: PMC2715025
- DOI: 10.1084/jem.20081605
M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis
Abstract
Antiangiogenic therapy for the treatment of cancer and other neovascular diseases is desired to be selective for pathological angiogenesis and lymphangiogenesis. Macrophage colony-stimulating factor (M-CSF), a cytokine required for the differentiation of monocyte lineage cells, promotes the formation of high-density vessel networks in tumors and therefore possesses therapeutic potential as an M-CSF inhibitor. However, the physiological role of M-CSF in vascular and lymphatic development, as well as the precise mechanisms underlying the antiangiogenic effects of M-CSF inhibition, remains unclear. Moreover, therapeutic potential of M-CSF inhibition in other neovascular diseases has not yet been evaluated. We used osteopetrotic (op/op) mice to demonstrate that M-CSF deficiency reduces the abundance of LYVE-1(+) and LYVE1(-) macrophages, resulting in defects in vascular and lymphatic development. In ischemic retinopathy, M-CSF was required for pathological neovascularization but was not required for the recovery of normal vasculature. In mouse osteosarcoma, M-CSF inhibition effectively suppressed tumor angiogenesis and lymphangiogenesis, and it disorganized extracellular matrices. In contrast to VEGF blockade, interruption of M-CSF inhibition did not promote rapid vascular regrowth. Continuous M-CSF inhibition did not affect healthy vascular and lymphatic systems outside tumors. These results suggest that M-CSF-targeted therapy is an ideal strategy for treating ocular neovascular diseases and cancer.
Figures
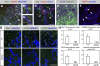




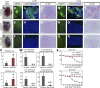

References
-
- Carmeliet P. 2005. Angiogenesis in life, disease and medicine.Nature. 438:932–936 - PubMed
-
- Ferrara N., Kerbel R.S. 2005. Angiogenesis as a therapeutic target.Nature. 438:967–974 - PubMed
-
- Rudge J.S., Thurston G., Davis S., Papadopoulos N., Gale N., Wiegand S.J., Yancopoulos G.D. 2005. VEGF trap as a novel antiangiogenic treatment currently in clinical trials for cancer and eye diseases, and VelociGene-based discovery of the next generation of angiogenesis targets.Cold Spring Harb. Symp. Quant. Biol. 70:411–418 - PubMed
-
- Pieramici D.J., Rabena M.D. 2008. Anti-VEGF therapy: comparison of current and future agents.Eye. 22:1330–1336 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

