Neurodevelopment genes in lampreys reveal trends for forebrain evolution in craniates
- PMID: 19399187
- PMCID: PMC2671401
- DOI: 10.1371/journal.pone.0005374
Neurodevelopment genes in lampreys reveal trends for forebrain evolution in craniates
Abstract
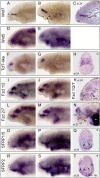
The forebrain is the brain region which has undergone the most dramatic changes through vertebrate evolution. Analyses conducted in lampreys are essential to gain insight into the broad ancestral characteristics of the forebrain at the dawn of vertebrates, and to understand the molecular basis for the diversifications that have taken place in cyclostomes and gnathostomes following their splitting. Here, we report the embryonic expression patterns of 43 lamprey genes, coding for transcription factors or signaling molecules known to be involved in cell proliferation, stemcellness, neurogenesis, patterning and regionalization in the developing forebrain. Systematic expression patterns comparisons with model organisms highlight conservations likely to reflect shared features present in the vertebrate ancestors. They also point to changes in signaling systems -pathways which control the growth and patterning of the neuroepithelium-, which may have been crucial in the evolution of forebrain anatomy at the origin of vertebrates.
Conflict of interest statement
Figures








References
-
- Ota KG, Kuratani S. The history of scientific endeavors towards understanding hagfish embryology. Zoolog Sci. 2006;23:403–418. - PubMed
-
- Osorio J, Rétaux S. The lamprey in evolutionary studies. Dev Genes Evol. 2008;218:221–235. - PubMed
-
- Kuratani S, Kuraku S, Murakami Y. Lamprey as an evo-devo model: lessons from comparative embryology and molecular phylogenetics. Genesis. 2002;34:175–183. - PubMed
-
- Bullock TH, Moore JK, Fields RD. Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48:145–148. - PubMed
-
- Butler AB, Hodos W. The forebrain: telencephalon. In: Butler AB, Hodos W, editors. Comparative vertebrate neuroanatomy. New York: Wiley-Liss; 1996. pp. 349–437.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

