Effect of levodopa priming on dopamine neuron transplant efficacy and induction of abnormal involuntary movements in parkinsonian rats
- PMID: 19399877
- PMCID: PMC2886671
- DOI: 10.1002/cne.22037
Effect of levodopa priming on dopamine neuron transplant efficacy and induction of abnormal involuntary movements in parkinsonian rats
Abstract
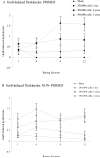
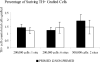
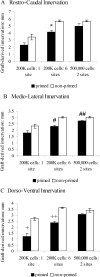
Clinical trials of neural grafting for Parkinson's disease (PD) have produced variable, but overall disappointing, results. One particular disappointment has been the development of aberrant motor complications following dopamine (DA) neuron grafting. Despite a lack of consistent benefit, the utility of dopamine neuron replacement remains supported by clinical and basic data. In a continued effort to elucidate factors that might improve this therapy, we used a parkinsonian rat model to examine whether pregraft chronic levodopa affected graft efficacy and/or graft-induced dyskinesia (GID) induction. Indeed, all grafted PD patients to date have had a pregraft history of long-term levodopa. It is well established that long-term levodopa results in a plethora of long-lasting neurochemical alterations and genomic changes indicative of altered structural and synaptic plasticity. Thus, therapeutic dopamine terminal replacement in a striatal environment complicated by such changes could be expected to lead to abnormal or inappropriate connections between graft and host brain and to contribute to suboptimal efficacy and/or postgraft GID behaviors. To investigate the effect of pregraft levodopa, one group of parkinsonian rats received levodopa for 4 weeks prior to grafting. A second levodopa-naïve group was grafted, and the grafts were allowed to mature for 9 weeks prior to introducing chronic levodopa. We report here that, in parkinsonian rats, preexposure to chronic levodopa significantly reduces behavioral and neurochemical efficacy of embryonic dopamine grafts. Furthermore, dopamine terminal replacement prior to introduction of chronic levodopa is highly effective at preventing development of levodopa-induced dyskinesias, and GID-like behaviors occur regardless of pregraft levodopa status.
Copyright 2009 Wiley-Liss, Inc.
Figures

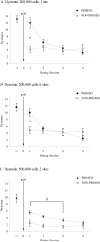
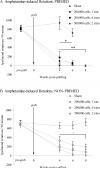
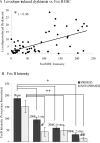
References
-
- Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol Dis. 1999;6:461–474. - PubMed
-
- Andersson M, Westin JE, Cenci MA. Time course of striatal DeltaFosB-like immunoreactivity and prodynorphin mRNA levels after discontinuation of chronic dopaminomimetic treatment. Eur J Neurosci. 2003;17:661–666. - PubMed
-
- Bolam JP, Izzo PN. The postsynaptic targets of substance P-immunoreactive terminals in the rat neostriatum with particular reference to identified spiny striatonigral neurons. Exp Brain Res. 1988;70:361–77. - PubMed
-
- Brown P, Williams D. Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol. 2005;116(11):2510–9. Review. - PubMed
-
- Caligiuri MP, Lohr JB. Worsening of postural tremor in patients with levodopa-induced dyskinesia: a quantitative analysis. Clin Neuropharmacol. 1993;16:244–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

