The brainstem and serotonin in the sudden infant death syndrome
- PMID: 19400695
- PMCID: PMC3268259
- DOI: 10.1146/annurev.pathol.4.110807.092322
The brainstem and serotonin in the sudden infant death syndrome
Abstract
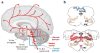
The sudden infant death syndrome (SIDS) is the sudden death of an infant under one year of age that is typically associated with sleep and that remains unexplained after a complete autopsy and death scene investigation. A leading hypothesis about its pathogenesis is that many cases result from defects in brainstem-mediated protective responses to homeostatic stressors occurring during sleep in a critical developmental period. Here we review the evidence for the brainstem hypothesis in SIDS with a focus upon abnormalities related to the neurotransmitter serotonin in the medulla oblongata, as these are the most robust pathologic findings to date. In this context, we synthesize the human autopsy data with genetic, whole-animal, and cellular data concerning the function and development of the medullary serotonergic system. These emerging data suggest an important underlying mechanism in SIDS that may help lead to identification of infants at risk and specific interventions to prevent death.
Figures





References
-
- Krous HF, Beckwith JB, Byard RW, Rognum TO, Bajanowski T, et al. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114:234–38. - PubMed
-
- Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics: 2004. Pediatrics. 2006;117:168–83. - PubMed
-
- Mathews TJ, MacDorman MF. Infant mortality statistics from the 2003 period linked birth/infant death data sets. Natl Vital Stat Rep. 2006;54:1–29. - PubMed
-
- Willinger M, Hoffman HJ, Hartford RB. Infant sleep position and risk for sudden infant death syndrome: report of meeting held January 13 and 14, 1994, National Institutes of Health, Bethesda, MD. Pediatrics. 1994;93:814–19. - PubMed
-
- Corwin MJ, Lesko SM, Heeren T, Vezina RM, Hunt CE, et al. Secular changes in sleep position during infancy: 1995–1998. Pediatrics. 2003;111:52–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

