Establishment of axon-dendrite polarity in developing neurons
- PMID: 19400726
- PMCID: PMC3170863
- DOI: 10.1146/annurev.neuro.31.060407.125536
Establishment of axon-dendrite polarity in developing neurons
Abstract
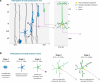
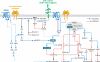
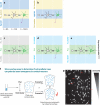
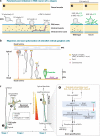
Neurons are among the most highly polarized cell types in the body, and the polarization of axon and dendrites underlies the ability of neurons to integrate and transmit information in the brain. Significant progress has been made in the identification of the cellular and molecular mechanisms underlying the establishment of neuronal polarity using primarily in vitro approaches such as dissociated culture of rodent hippocampal and cortical neurons. This model has led to the predominant view suggesting that neuronal polarization is specified largely by stochastic, asymmetric activation of intracellular signaling pathways. Recent evidence shows that extracellular cues can play an instructive role during neuronal polarization in vitro and in vivo. In this review, we synthesize the recent data supporting an integrative model whereby extracellular cues orchestrate the intracellular signaling underlying the initial break of neuronal symmetry leading to axon-dendrite polarization.
Figures

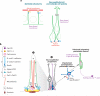
References
-
- Aravamudan B, Broadie K. Synaptic Drosophila UNC-13 is regulated by antagonistic G-protein pathways via a proteasome-dependent degradation mechanism. J Neurobiol. 2003;54:417–38. - PubMed
-
- Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. - PubMed
-
- Arimura N, Menager C, Fukata Y, Kaibuchi K. Role of CRMP-2 in neuronal polarity. J Neurobiol. 2004;58:34–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

