Diversity of sesquiterpene synthases in the basidiomycete Coprinus cinereus
- PMID: 19400802
- PMCID: PMC2723806
- DOI: 10.1111/j.1365-2958.2009.06717.x
Diversity of sesquiterpene synthases in the basidiomycete Coprinus cinereus
Abstract
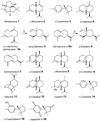
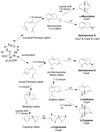
Fungi are a rich source of bioactive secondary metabolites, and mushroom-forming fungi (Agaricomycetes) are especially known for the synthesis of numerous bioactive and often cytotoxic sesquiterpenoid secondary metabolites. Compared with the large number of sesquiterpene synthases identified in plants, less than a handful of unique sesquiterpene synthases have been described from fungi. Here we describe the functional characterization of six sesquiterpene synthases (Cop1 to Cop6) and two terpene-oxidizing cytochrome P450 monooxygenases (Cox1 and Cox2) from Coprinus cinereus. The genes were cloned and, except for cop5, functionally expressed in Escherichia coli and/or Saccharomyces cerevisiae. Cop1 and Cop2 each synthesize germacrene A as the major product. Cop3 was identified as an alpha-muurolene synthase, an enzyme that has not been described previously, while Cop4 synthesizes delta-cadinene as its major product. Cop6 was originally annotated as a trichodiene synthase homologue but instead was found to catalyse the highly specific synthesis of alpha-cuprenene. Coexpression of cop6 and the two monooxygenase genes next to it yields oxygenated alpha-cuprenene derivatives, including cuparophenol, suggesting that these genes encode the enzymes for the biosynthesis of antimicrobial quinone sesquiterpenoids (known as lagopodins) that were previously isolated from C. cinereus and other Coprinus species.
Figures




Similar articles
-
Selectivity of fungal sesquiterpene synthases: role of the active site's H-1 alpha loop in catalysis.Appl Environ Microbiol. 2010 Dec;76(23):7723-33. doi: 10.1128/AEM.01811-10. Epub 2010 Oct 1. Appl Environ Microbiol. 2010. PMID: 20889795 Free PMC article.
-
Sesquiterpene synthases Cop4 and Cop6 from Coprinus cinereus: catalytic promiscuity and cyclization of farnesyl pyrophosphate geometric isomers.Chembiochem. 2010 May 17;11(8):1093-106. doi: 10.1002/cbic.200900671. Chembiochem. 2010. PMID: 20419721 Free PMC article.
-
Biosynthesis of lagopodins in mushroom involves a complex network of oxidation reactions.Org Biomol Chem. 2019 Jan 2;17(2):234-239. doi: 10.1039/c8ob02814a. Org Biomol Chem. 2019. PMID: 30556075
-
MTPSLs: New Terpene Synthases in Nonseed Plants.Trends Plant Sci. 2018 Feb;23(2):121-128. doi: 10.1016/j.tplants.2017.09.014. Epub 2017 Oct 20. Trends Plant Sci. 2018. PMID: 29066043 Review.
-
Applications of dimethylallyltryptophan synthases and other indole prenyltransferases for structural modification of natural products.Appl Microbiol Biotechnol. 2009 Sep;84(4):631-9. doi: 10.1007/s00253-009-2128-z. Epub 2009 Jul 25. Appl Microbiol Biotechnol. 2009. PMID: 19633837 Review.
Cited by
-
Identification of a fungal 1,8-cineole synthase from Hypoxylon sp. with specificity determinants in common with the plant synthases.J Biol Chem. 2015 Mar 27;290(13):8511-26. doi: 10.1074/jbc.M114.636159. Epub 2015 Feb 3. J Biol Chem. 2015. PMID: 25648891 Free PMC article.
-
Genome sequence of the model medicinal mushroom Ganoderma lucidum.Nat Commun. 2012 Jun 26;3:913. doi: 10.1038/ncomms1923. Nat Commun. 2012. PMID: 22735441 Free PMC article.
-
Colocalization of amanitin and a candidate toxin-processing prolyl oligopeptidase in Amanita basidiocarps.Eukaryot Cell. 2010 Dec;9(12):1891-900. doi: 10.1128/EC.00161-10. Epub 2010 Oct 1. Eukaryot Cell. 2010. PMID: 20889720 Free PMC article.
-
RNA-seq discovery, functional characterization, and comparison of sesquiterpene synthases from Solanum lycopersicum and Solanum habrochaites trichomes.Plant Mol Biol. 2011 Nov;77(4-5):323-36. doi: 10.1007/s11103-011-9813-x. Epub 2011 Aug 5. Plant Mol Biol. 2011. PMID: 21818683 Free PMC article.
-
Genomic Features of Taiwanofungus gaoligongensis and the Transcriptional Regulation of Secondary Metabolite Biosynthesis.J Fungi (Basel). 2024 Nov 27;10(12):826. doi: 10.3390/jof10120826. J Fungi (Basel). 2024. PMID: 39728323 Free PMC article.
References
-
- Abraham WR. Bioactive sesquiterpenes produced by fungi: are they useful for humans as well? Curr Med Chem. 2001;8:583–606. - PubMed
-
- Bertea CM, Voster A, Verstappen FW, Maffei M, Beekwilder J, Bouwmeester HJ. Isoprenoid biosynthesis in Artemisia annua: cloning and heterologous expression of a germacrene A synthase from a glandular trichome cDNA library. Arch Biochem Biophys. 2006;448:3–12. - PubMed
-
- Bottom CB, Siehr DJ. Hydroxylagopodin B: Sesquiterpenoid quinone from a mutant strain of Coprinus macrorhizus var microsporus. Phytochemistry. 1975;14:1433–1433.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

