Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo-controlled feasibility study
- PMID: 19400893
- PMCID: PMC2728895
- DOI: 10.1111/j.1365-2222.2009.03187.x
Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo-controlled feasibility study
Abstract
Background: Epidemiological evidence suggests that hookworm infection protects against asthma. However, for ethical and safety reasons, before testing this hypothesis in a clinical trial in asthma it is necessary to establish whether experimental hookworm infection might exacerbate airway responsiveness during larval lung migration.
Objective: To determine whether hookworm larval migration through the lungs increases airway responsiveness in allergic individuals with measurable airway responsiveness but not clinical asthma, and investigate the general tolerability of infection and effect on allergic symptoms.
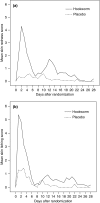
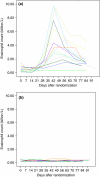
Methods: Thirty individuals with allergic rhinoconjunctivitis and measurable airway responsiveness to adenosine monophosphate (AMP) but not clinically diagnosed asthma were randomized, double-blind to cutaneous administration of either 10 hookworm larvae or histamine placebo, and followed for 12 weeks. The primary outcome was the maximum fall from baseline in provocative dose of inhaled AMP required to reduce 1-s forced expiratory volume by 10% (PD(10)AMP) measured at any time over the 4 weeks after active or placebo infection. Secondary outcomes included peak flow variability in the 4 weeks after infection, rhinoconjunctivitis symptom severity and adverse effect diary scores over the 12-week study period, and change in allergen skin test responses between baseline and 12 weeks.
Results: Mean maximum change in PD(10)AMP from baseline was slightly but not significantly greater in the hookworm than the placebo group (-1.67 and -1.16 doubling doses; mean difference -0.51, 95% confidence interval -1.80 to 0.78, P=0.42). Symptom scores of potential adverse effects were more commonly reported in the hookworm group, but infection was generally well tolerated. There were no significant differences in peak-flow variability, rhinoconjunctivitis symptoms or skin test responses between groups.
Conclusion: Hookworm infection did not cause clinically significant exacerbation of airway responsiveness and was well tolerated. Suitably powered trials are now indicated to determine the clinical effectiveness of hookworm infection in allergic rhinoconjunctivitis and asthma.
Figures
References
-
- Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32. - PubMed
-
- Leonardi-Bee J, Pritchard D, Britton J. Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am J Respir Crit Care Med. 2006;174:514–23. - PubMed
-
- Cooper PJ, Chico ME, Rodrigues LC, et al. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J Allergy Clin Immunol. 2003;111:995–1000. - PubMed
-
- Davey G, Venn A, Belete H, Berhane Y, Britton J. Wheeze, allergic sensitization and geohelminth infection in Butajira, Ethiopia. Clin Exp Allergy. 2005;35:301–7. - PubMed
-
- Scrivener S, Yemaneberhan H, Zebenigus M, et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case–control study. Lancet. 2001;358:1493–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

