Activation of p53-regulated pro-apoptotic signaling pathways in PrP-mediated myopathy
- PMID: 19400950
- PMCID: PMC2683871
- DOI: 10.1186/1471-2164-10-201
Activation of p53-regulated pro-apoptotic signaling pathways in PrP-mediated myopathy
Abstract
Background: We have reported that doxycycline-induced over-expression of wild type prion protein (PrP) in skeletal muscles of Tg(HQK) mice is sufficient to cause a primary myopathy with no signs of peripheral neuropathy. The preferential accumulation of the truncated PrP C1 fragment was closely correlated with these myopathic changes. In this study we use gene expression profiling to explore the temporal program of molecular changes underlying the PrP-mediated myopathy.
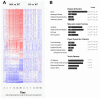
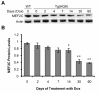
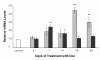
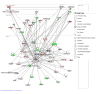
Results: We used DNA microarrays, and confirmatory real-time PCR and Western blot analysis to demonstrate deregulation of a large number of genes in the course of the progressive myopathy in the skeletal muscles of doxycycline-treated Tg(HQK) mice. These include the down-regulation of genes coding for the myofibrillar proteins and transcription factor MEF2c, and up-regulation of genes for lysosomal proteins that is concomitant with increased lysosomal activity in the skeletal muscles. Significantly, there was prominent up-regulation of p53 and p53-regulated genes involved in cell cycle arrest and promotion of apoptosis that paralleled the initiation and progression of the muscle pathology.
Conclusion: The data provides the first in vivo evidence that directly links p53 to a wild type PrP-mediated disease. It is evident that several mechanistic features contribute to the myopathy observed in PrP over-expressing mice and that p53-related apoptotic pathways appear to play a major role.
Figures



Similar articles
-
Inducible overexpression of wild-type prion protein in the muscles leads to a primary myopathy in transgenic mice.Proc Natl Acad Sci U S A. 2007 Apr 17;104(16):6800-5. doi: 10.1073/pnas.0608885104. Epub 2007 Apr 9. Proc Natl Acad Sci U S A. 2007. PMID: 17420473 Free PMC article.
-
Molecular mechanisms of nutlin-induced apoptosis in multiple myeloma: evidence for p53-transcription-dependent and -independent pathways.Cancer Biol Ther. 2010 Sep 15;10(6):567-78. doi: 10.4161/cbt.10.6.12535. Epub 2010 Oct 1. Cancer Biol Ther. 2010. PMID: 20595817 Free PMC article.
-
The cellular prion protein (PrPC) prevents apoptotic neuronal cell death and mitochondrial dysfunction induced by serum deprivation.Brain Res Mol Brain Res. 2004 Apr 29;124(1):40-50. doi: 10.1016/j.molbrainres.2004.02.005. Brain Res Mol Brain Res. 2004. PMID: 15093684
-
The P53 pathway: what questions remain to be explored?Cell Death Differ. 2006 Jun;13(6):1027-36. doi: 10.1038/sj.cdd.4401910. Cell Death Differ. 2006. PMID: 16557269 Review.
-
The roles of PARK gene family in myopathy.Yi Chuan. 2022 Jul 20;44(7):545-555. doi: 10.16288/j.yczz.22-105. Yi Chuan. 2022. PMID: 35858767 Review.
Cited by
-
Lack of a-disintegrin-and-metalloproteinase ADAM10 leads to intracellular accumulation and loss of shedding of the cellular prion protein in vivo.Mol Neurodegener. 2011 May 27;6:36. doi: 10.1186/1750-1326-6-36. Mol Neurodegener. 2011. PMID: 21619641 Free PMC article.
-
Cellular prion protein dysfunction in a prototypical inherited metabolic myopathy.Cell Mol Life Sci. 2021 Mar;78(5):2157-2167. doi: 10.1007/s00018-020-03624-6. Epub 2020 Sep 1. Cell Mol Life Sci. 2021. PMID: 32875355 Free PMC article.
-
Neuroprotective effect and potential of cellular prion protein and its cleavage products for treatment of neurodegenerative disorders part II: strategies for therapeutics development.Expert Rev Neurother. 2021 Sep;21(9):983-991. doi: 10.1080/14737175.2021.1965882. Epub 2021 Sep 2. Expert Rev Neurother. 2021. PMID: 34470554 Free PMC article.
-
Neuroprotective effect and potential of cellular prion protein and its cleavage products for treatment of neurodegenerative disorders part I. a literature review.Expert Rev Neurother. 2021 Sep;21(9):969-982. doi: 10.1080/14737175.2021.1965881. Epub 2021 Sep 2. Expert Rev Neurother. 2021. PMID: 34470561 Free PMC article. Review.
-
The Cellular Prion Protein and the Hallmarks of Cancer.Cancers (Basel). 2021 Oct 8;13(19):5032. doi: 10.3390/cancers13195032. Cancers (Basel). 2021. PMID: 34638517 Free PMC article. Review.
References
-
- Diarra-Mehrpour M, Arrabal S, Jalil A, Pinson X, Gaudin C, Pietu G, Pitaval A, Ripoche H, Eloit M, Dormont D, Chouaib S. Prion protein prevents human breast carcinoma cell line from tumor necrosis factor alpha-induced cell death. Cancer Res. 2004;64:719–727. doi: 10.1158/0008-5472.CAN-03-1735. - DOI - PubMed
-
- Paitel E, Sunyach C, Alves da Costa C, Bourdon JC, Vincent B, Checler F. Primary cultured neurons devoid of cellular prion display lower responsiveness to staurosporine through the control of p53 at both transcriptional and post-transcriptional levels. J Biol Chem. 2004;279:612–618. doi: 10.1074/jbc.M310453200. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

