Protocol for the Proactive Or Reactive Telephone Smoking CeSsation Support (PORTSSS) trial
- PMID: 19400961
- PMCID: PMC2679731
- DOI: 10.1186/1745-6215-10-26
Protocol for the Proactive Or Reactive Telephone Smoking CeSsation Support (PORTSSS) trial
Abstract
Background: Telephone quit lines are accessible to many smokers and are used to engage motivated smokers to make quit attempts. Smoking cessation counselling provided via telephone can either be reactive (i.e. primarily involving the provision of evidence-based information), or proactive (i.e. primarily involving repeated, sequenced calls from and interaction with trained cessation counsellors). Some studies have found proactive telephone counselling more effective and this trial will investigate whether or not proactive telephone support for smoking cessation, delivered through the National Health Service (NHS) Smoking Helpline is more effective or cost-effective than reactive support. It will also investigate whether or not providing nicotine replacement therapy (NRT), in addition to telephone counselling, has an adjunctive impact on smoking cessation rates and whether or not this is cost effective.
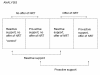
Methods: This will be a parallel group, factorial design RCT, conducted through the English national NHS Smoking Helpline which is run from headquarters in Glasgow. Participants will be smokers who call the helpline from any location in England and who wish to stop smoking. If 644 participants are recruited to four equally-sized trial groups (total sample size = 2576), the trial will have 90% power for detecting a treatment effect (Odds Ratio) of 1.5 for each of the two interventions: i) proactive versus reactive support and ii) the offer of NRT versus no offer. The primary outcome measure for the study is self-reported, prolonged abstinence from smoking for at least six months following an agreed quit date. A concurrent health economic evaluation will investigate the cost effectiveness of the two interventions when delivered via a telephone helpline.
Discussion: The PORTSSS trial will provide high quality evidence to determine the most appropriate kind of counselling which should be provided via the NHS Smoking Helpline and also whether or not an additional offer of cost-free NRT is effective and cost effective for smoking cessation.
Trial registration: ClinicalTrials.gov NCT00775944.
Figures
References
-
- Tobacco Advisory Group of the Royal College of Physicians . Nicotine addiction in Britain. London: Royal College of Physicians of London; 2000.
-
- Tobacco Advisory Group of the Royal College of Physicians . Going smoke-free: The case for clean air in the home, at work and in public places. London: Royal College of Physicians of London; 2005.
-
- Silagy C. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2000. p. CD000146. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous