Cellular responses to DNA double-strand breaks after low-dose gamma-irradiation
- PMID: 19401436
- PMCID: PMC2709554
- DOI: 10.1093/nar/gkp237
Cellular responses to DNA double-strand breaks after low-dose gamma-irradiation
Abstract
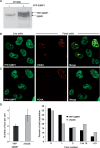
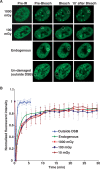
DNA double-strand breaks (DSBs) are a serious threat to genome stability and cell viability. Although biological effects of low levels of radiation are not clear, the risks of low-dose radiation are of societal importance. Here, we directly monitored induction and repair of single DSBs and quantitatively analyzed the dynamics of interaction of DNA repair proteins at individual DSB sites in living cells using 53BP1 fused to yellow fluorescent protein (YFP-53BP1) as a surrogate marker. The number of DSBs formed was linear with dose from 5 mGy to 1 Gy. The DSBs induced by very low radiation doses (5 mGy) were repaired with efficiency similar to repair of DSBs induced at higher doses. The YFP-53BP1 foci are dynamic structures: 53BP1 rapidly and reversibly interacted at these DSB sites. The time frame of recruitment and affinity of 53BP1 for DSB sites were indistinguishable between low and high doses, providing mechanistic evidence for the similar DSB repair after low- and high-dose radiation. These findings have important implications for estimating the risk associated with low-dose radiation exposure on human health.
Figures




References
-
- Prasad KN, Cole WC, Hasse GM. Health risks of low dose ionizing radiation in humans: a review. Exp. Biol. Med. 2004;229:378–382. - PubMed
-
- Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat. Res. 2003;159:567–580. - PubMed
-
- Strzelczyk JJ, Damilakis J, Marx MV, Macura KJ. Facts and controversies about radiation exposure, part 2: low-level exposures and cancer risk. J. Am. Coll. Radiol. 2007;4:32–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

