Four-gene expression ratio test for survival in patients undergoing surgery for mesothelioma
- PMID: 19401544
- PMCID: PMC2677573
- DOI: 10.1093/jnci/djp061
Four-gene expression ratio test for survival in patients undergoing surgery for mesothelioma
Abstract
Background: Malignant pleural mesothelioma has few effective treatments, one being cytoreductive surgery. We previously developed a gene ratio test to predict outcome of malignant pleural mesothelioma patients undergoing surgery. In this study, we investigated the predictive value and technical assay performance of this test in patients with malignant pleural mesothelioma.
Methods: Clinical data were obtained prospectively from 120 consecutive patients with malignant pleural mesothelioma who were scheduled for debulking surgery at one institution. Specimens were obtained at surgery or by pleural biopsy examination. Expression data for four genes were collected from tumor specimens, and three ratios of gene expression (TM4SF1/PKM2, TM4SF1/ARHGDIA, and COBLL1/ARHGDIA) were determined by quantitative reverse transcriptase-polymerase chain reaction. Patients were assigned to good or poor outcome groups by the gene ratio test. Survival was estimated by the Kaplan-Meier method and the log-rank test in univariate analyses. A multivariable Cox proportional hazards model was used to control for prognostic factors. Technical robustness was determined by using up to 30 specimens per patient, two biopsy techniques, and two performance sites. All statistical tests were two-sided.
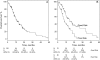
Results: The test predicted overall survival (P < .001) and cancer-specific survival (P = .007) in univariate analysis and overall survival in multivariable analysis (hazard ratio for death = 2.09, 95% confidence interval [CI] = 1.27 to 3.45, P = .004). The test was reproducible within patients and repeatable between two determinations for specimens with widely varying tumor cell contents. Repeatability between two determinations was 88.5% (95% CI = 84.0% to 92.2%) or, when technically unacceptable test values were excluded, 91.9% (95% CI = 87.4% to 95.1%). Reproducibility between two determinations was 96.1% (95% CI = 86.5% to 99.5%). Combining the gene ratio test and other prognostic factors allowed prospective discrimination between patients at high risk (median survival = 6.9 months, 95% CI = 2.6 to 8.9 months; 3-year survival = 0%) and low risk (median survival = 31.9 months, 95% CI = 21.9 to 41.7 months; 3-year survival = 42%).
Conclusion: The gene ratio test for survival of patients with malignant pleural mesothelioma has robust predictive value and technical assay performance.
Figures

References
-
- Chang MY, Sugarbaker DJ. Extrapleural pneumonectomy for diffuse malignant pleural mesothelioma: techniques and complications. Thorac Surg Clin. 2004;14(4):523–530. - PubMed
-
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–2644. - PubMed
-
- Sugarbaker D, Strauss GM, Lynch TJ, et al. Node status has prognostic significance in the multimodality therapy of diffuse, malignant mesothelioma. J Clin Oncol. 1993;11(6):1172–1178. - PubMed
-
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999;117(1):54–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

