Clusterin, a haploinsufficient tumor suppressor gene in neuroblastomas
- PMID: 19401549
- PMCID: PMC2720718
- DOI: 10.1093/jnci/djp063
Clusterin, a haploinsufficient tumor suppressor gene in neuroblastomas
Abstract
Background: Clusterin expression in various types of human cancers may be higher or lower than in normal tissue, and clusterin may promote or inhibit apoptosis, cell motility, and inflammation. We investigated the role of clusterin in tumor development in mouse models of neuroblastoma.
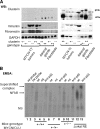
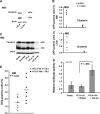
Methods: We assessed expression of microRNAs in the miR-17-92 cluster by real-time reverse transcription-polymerase chain reaction in MYCN-transfected SH-SY5Y and SH-EP cells and inhibited expression by transfection with microRNA antisense oligonucleotides. Tumor development was studied in mice (n = 66) that were heterozygous or homozygous for the MYCN transgene and/or for the clusterin gene; these mice were from a cross between MYCN-transgenic mice, which develop neuroblastoma, and clusterin-knockout mice. Tumor growth and metastasis were studied in immunodeficient mice that were injected with human neuroblastoma cells that had enhanced (by clusterin transfection, four mice per group) or reduced (by clusterin short hairpin RNA [shRNA] transfection, eight mice per group) clusterin expression. All statistical tests were two-sided.
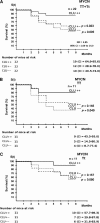
Results: Clusterin expression increased when expression of MYCN-induced miR-17-92 microRNA cluster in SH-SY5Y neuroblastoma cells was inhibited by transfection with antisense oligonucleotides compared with scrambled oligonucleotides. Statistically significantly more neuroblastoma-bearing MYCN-transgenic mice were found in groups with zero or one clusterin allele than in those with two clusterin alleles (eg, 12 tumor-bearing mice in the zero-allele group vs three in the two-allele group, n = 22 mice per group; relative risk for neuroblastoma development = 4.85, 95% confidence interval [CI] = 1.69 to 14.00; P = .005). Five weeks after injection, fewer clusterin-overexpressing LA-N-5 human neuroblastoma cells than control cells were found in mouse liver or bone marrow, but statistically significantly more clusterin shRNA-transfected HTLA230 cells (3.27%, with decreased clusterin expression) than control-transfected cells (1.53%) were found in the bone marrow (difference = 1.74%, 95% CI = 0.24% to 3.24%, P = .026).
Conclusions: We report, to our knowledge, the first genetic evidence that clusterin is a tumor and metastasis suppressor gene.
Figures




Similar articles
-
Effects of MYCN antisense oligonucleotide administration on tumorigenesis in a murine model of neuroblastoma.J Natl Cancer Inst. 2003 Sep 17;95(18):1394-403. doi: 10.1093/jnci/djg045. J Natl Cancer Inst. 2003. PMID: 13130115
-
ABCC multidrug transporters in childhood neuroblastoma: clinical and biological effects independent of cytotoxic drug efflux.J Natl Cancer Inst. 2011 Aug 17;103(16):1236-51. doi: 10.1093/jnci/djr256. Epub 2011 Jul 28. J Natl Cancer Inst. 2011. PMID: 21799180 Free PMC article.
-
MYCN and MYC regulate tumor proliferation and tumorigenesis directly through BMI1 in human neuroblastomas.FASEB J. 2011 Dec;25(12):4138-49. doi: 10.1096/fj.11-185033. Epub 2011 Aug 19. FASEB J. 2011. PMID: 21856782 Free PMC article.
-
A genome-wide search for promoters that respond to increased MYCN reveals both new oncogenic and tumor suppressor microRNAs associated with aggressive neuroblastoma.Cancer Res. 2011 Jun 1;71(11):3841-51. doi: 10.1158/0008-5472.CAN-10-4391. Epub 2011 Apr 15. Cancer Res. 2011. PMID: 21498633
-
MicroRNA involvement in the pathogenesis of neuroblastoma: potential for microRNA mediated therapeutics.Curr Pharm Des. 2009;15(4):456-62. doi: 10.2174/138161209787315837. Curr Pharm Des. 2009. PMID: 19199973 Free PMC article. Review.
Cited by
-
Targeting of TGFβ signature and its essential component CTGF by miR-18 correlates with improved survival in glioblastoma.RNA. 2013 Feb;19(2):177-90. doi: 10.1261/rna.036467.112. Epub 2012 Dec 18. RNA. 2013. PMID: 23249750 Free PMC article.
-
HBX Protein-Induced Downregulation of microRNA-18a is Responsible for Upregulation of Connective Tissue Growth Factor in HBV Infection-Associated Hepatocarcinoma.Med Sci Monit. 2016 Jul 16;22:2492-500. doi: 10.12659/msm.895943. Med Sci Monit. 2016. PMID: 27421245 Free PMC article.
-
Role of the CASZ1 transcription factor in tissue development and disease.Eur J Med Res. 2023 Dec 5;28(1):562. doi: 10.1186/s40001-023-01548-y. Eur J Med Res. 2023. PMID: 38053207 Free PMC article. Review.
-
Identification of CASZ1 NES reveals potential mechanisms for loss of CASZ1 tumor suppressor activity in neuroblastoma.Oncogene. 2017 Jan 5;36(1):97-109. doi: 10.1038/onc.2016.179. Epub 2016 Jun 6. Oncogene. 2017. PMID: 27270431 Free PMC article.
-
Extracellular clusterin limits the uptake of α-synuclein fibrils by murine and human astrocytes.Glia. 2021 Mar;69(3):681-696. doi: 10.1002/glia.23920. Epub 2020 Oct 12. Glia. 2021. PMID: 33045109 Free PMC article.
References
-
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3(3):203–216. - PubMed
-
- Lonergan GJ, Schwab CM, Suarez ES, Carlson CL. Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologic-pathologic correlation. Radiographics. 2002;22(4):911–934. - PubMed
-
- Shannan B, Seifert M, Leskov K, et al. Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ. 2006;13(1):12–19. - PubMed
-
- Trougakos IP, Gonos ES. Clusterin/apolipoprotein J in human aging and cancer. Int J Biochem Cell Biol. 2002;34(11):1430–1448. - PubMed
-
- Nizard P, Tetley S, Le Drean Y, et al. Stress-induced retrotranslocation of clusterin/ApoJ into the cytosol. Traffic. 2007;8(5):554–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

