SCF induces gamma-globin gene expression by regulating downstream transcription factor COUP-TFII
- PMID: 19401563
- PMCID: PMC2927201
- DOI: 10.1182/blood-2008-07-170712
SCF induces gamma-globin gene expression by regulating downstream transcription factor COUP-TFII
Abstract
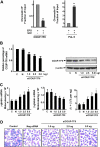
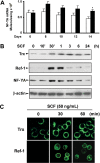
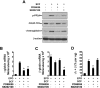
Increased fetal hemoglobin expression in adulthood is associated with acute stress erythropoiesis. However, the mechanisms underlying gamma-globin induction during the rapid expansion of adult erythroid progenitor cells have not been fully elucidated. Here, we examined COUP-TFII as a potential repressor of gamma-globin gene after stem cell factor (SCF) stimulation in cultured human adult erythroid progenitor cells. We found that COUP-TFII expression is suppressed by SCF through phosphorylation of serine/threonine phosphatase (PP2A) and correlated well with fetal hemoglobin induction. Furthermore, down-regulation of COUP-TFII expression with small interfering RNA (siRNA) significantly increases the gamma-globin expression during the erythroid maturation. Moreover, SCF-increased expression of NF-YA associated with redox regulator Ref-1 and cellular reducing condition enhances the effect of SCF on gamma-globin expression. Activation of Erk1/2 plays a critical role in SCF modulation of downstream transcriptional factor COUP-TFII, which is involved in the regulation of gamma-globin gene induction. Our data show that SCF stimulates Erk1/2 MAPK signaling pathway, which regulates the downstream repressor COUP-TFII by inhibiting serine/threonine phosphatase 2A activity, and that decreased COUP-TFII expression resulted in gamma-globin reactivation in adult erythropoiesis. These observations provide insight into the molecular pathways that regulate gamma-globin augmentation during stress erythropoiesis.
Figures




References
-
- Vakoc CR, Letting DL, Gheldof N, et al. Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol Cell. 2005;17:453–462. - PubMed
-
- Boyer SH, Belding TK, Margolet L, Noyes AN. Fetal hemoglobin restriction to a few erythrocytes (F cells) in normal human adults. Science. 1975;188:361–363. - PubMed
-
- Alter BP. Fetal erythropoiesis in stress hematopoiesis. Exp Hematol. 1979;7:200–209. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

