Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway
- PMID: 19401592
- PMCID: PMC2696635
- DOI: 10.1126/scisignal.2000188
Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway
Abstract
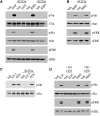
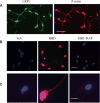
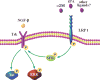
Low-density lipoprotein receptor-related protein 1 (LRP1) functions in endocytosis and intracellular signaling for a variety of structurally diverse ligands. Although LRP1 has been implicated in several aspects of neuronal function, molecular mechanisms underlying the activity of neuronal LRP1 remain unclear. Here, we describe a signaling pathway whereby LRP1 transactivates Trk receptors. Binding of tissue-type plasminogen activator or alpha(2)-macroglobulin (alpha(2)M) to LRP1 resulted in Src family kinase (SFK) activation and SFK-dependent Trk receptor transactivation in PC12 cells and neurons. Trk receptor transactivation was necessary for activation of Akt and extracellular signal-regulated kinase and for neurite outgrowth downstream of LRP1. Injection of the LRP1-binding domain of alpha(2)M into rat dorsal root ganglia induced Trk receptor phosphorylation, which was blocked by receptor-associated protein, an antagonist of ligand binding to LRP1. Trk receptor transactivation provides a mechanism by which diverse LRP1 ligands may show neurotrophic activity.
Figures






References
-
- Strickland DK, Gonias SL, Argraves WS. Diverse roles for the LDL receptor family. Trends Endocrinol Metab. 2002;13:66–74. - PubMed
-
- Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–2127. - PubMed
-
- Mantuano E, Inoue G, Li X, Takahashi K, Gaultier A, Gonias SL, Campana WM. The hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of schwann cells by binding to low-density lipoprotein receptor-related protein. J Neurosci. 2008;28:11571–11582. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

