Neurotransmitters drive combinatorial multistate postsynaptic density networks
- PMID: 19401593
- PMCID: PMC3280897
- DOI: 10.1126/scisignal.2000102
Neurotransmitters drive combinatorial multistate postsynaptic density networks
Abstract
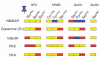
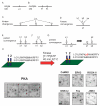
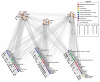
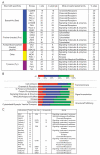
The mammalian postsynaptic density (PSD) comprises a complex collection of approximately 1100 proteins. Despite extensive knowledge of individual proteins, the overall organization of the PSD is poorly understood. Here, we define maps of molecular circuitry within the PSD based on phosphorylation of postsynaptic proteins. Activation of a single neurotransmitter receptor, the N-methyl-D-aspartate receptor (NMDAR), changed the phosphorylation status of 127 proteins. Stimulation of ionotropic and metabotropic glutamate receptors and dopamine receptors activated overlapping networks with distinct combinatorial phosphorylation signatures. Using peptide array technology, we identified specific phosphorylation motifs and switching mechanisms responsible for the integration of neurotransmitter receptor pathways and their coordination of multiple substrates in these networks. These combinatorial networks confer high information-processing capacity and functional diversity on synapses, and their elucidation may provide new insights into disease mechanisms and new opportunities for drug discovery.
Figures

References
-
- Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. - PubMed
-
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 2007;23:613–643. - PubMed
-
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. - PubMed
-
- Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, Choudhary JS, Grant SG. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J. Neurochem. 2006;97:16–23. - PubMed
-
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat. Neurosci. 2000;3:661–669. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

