Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury
- PMID: 19401709
- PMCID: PMC2846547
- DOI: 10.1038/jcbfm.2009.46
Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury
Abstract
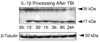
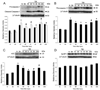
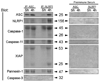
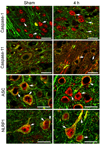
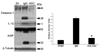
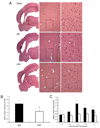
Traumatic brain injury elicits acute inflammation that in turn exacerbates primary brain damage. A crucial part of innate immunity in the immune privileged central nervous system involves production of proinflammatory cytokines mediated by inflammasome signaling. Here, we show that the nucleotide-binding, leucine-rich repeat pyrin domain containing protein 1 (NLRP1) inflammasome consisting of NLRP1, caspase-1, caspase-11, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), the X-linked inhibitor of apoptosis protein, and pannexin 1 is expressed in neurons of the cerebral cortex. Moderate parasagittal fluid-percussion injury (FPI) induced processing of interleukin-1beta, activation of caspase-1, cleavage of X-linked inhibitor of apoptosis protein, and promoted assembly of the NLRP1 inflammasome complex. Anti-ASC neutralizing antibodies administered immediately after fluid-percussion injury to injured rats reduced caspase-1 activation, X-linked inhibitor of apoptosis protein cleavage, and processing of interleukin-1beta, resulting in a significant decrease in contusion volume. These studies show that the NLRP1 inflammasome constitutes an important component of the innate central nervous system inflammatory response after traumatic brain injury and may be a novel therapeutic target for reducing the damaging effects of posttraumatic brain inflammation.
Figures
References
-
- Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29:534–544. - PubMed
-
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. - PubMed
-
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. - PubMed
-
- Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat Rev Drug Discov. 2004;3:330–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

