Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate
- PMID: 19401766
- PMCID: PMC2670536
- DOI: 10.1371/journal.pone.0005388
Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate
Abstract
Background: Understanding how the mechanical microenvironment influences cell fate, and more importantly, by what molecular mechanisms, will enhance not only the knowledge of mesenchymal stem cell biology but also the field of regenerative medicine. Mechanical stimuli, specifically loading induced oscillatory fluid flow, plays a vital role in promoting healthy bone development, homeostasis and morphology. Recent studies suggest that such loading induced fluid flow has the potential to regulate osteogenic differentiation via the upregulation of multiple osteogenic genes; however, the molecular mechanisms involved in the transduction of a physical signal into altered cell fate have yet to be determined.
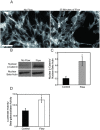
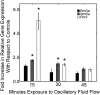
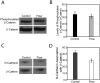
Methods and principal findings: Using immuno-staining, western blot analysis and luciferase assays, we demonstrate the oscillatory fluid flow regulates beta-catenin nuclear translocation and gene transcription. Additionally, real time RT-PCR analysis suggests that flow induces Wnt5a and Ror2 upregulation, both of which are essential for activating the small GTPase, RhoA, upon flow exposure. Furthermore, although beta-catenin phosphorylation is not altered by flow, its association with N-cadherin is, indicating that flow-induced beta-catenin signaling is initiated by adherens junction signaling.
Conclusion: We propose that the mechanical microenvironment of bone has the potential to regulate osteogenic differentiation by initiating multiple key molecular pathways that are essential for such lineage commitment. Specifically, non-canonical Wnt5a signaling involving Ror2 and RhoA as well as N-cadherin mediated beta-catenin signaling are necessary for mechanically induced osteogenic differentiation.
Conflict of interest statement
Figures
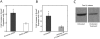

References
-
- Turner CH. Bone strength: current concepts. Ann N Y Acad Sci. 2006;1068:429–46. - PubMed
-
- Carter DR, Beaupre GS, Giori NJ, Helms JA. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998;355:S41–55. - PubMed
-
- Carter DR, Orr TE, Fyhrie DP. Relationships between loading history and femoral cancellous bone architecture. J Biomech. 1989;22:231–44. - PubMed
-
- Brown TD, Pedersen DR, Gray ML, Brand RA, Rubin CT. Toward an identification of mechanical parameters initiating periosteal remodeling: a combined experimental and analytic approach. J Biomech. 1990;23:893–905. - PubMed
-
- Ruff C, Holt B, Trinkaus E. Who's afraid of the big bad Wolff?: “Wolff's law” and bone functional adaptation. Am J Phys Anthropol. 2006;129:484–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

