Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides
- PMID: 19402754
- PMCID: PMC2671563
- DOI: 10.1371/journal.pbio.1000097
Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides
Abstract
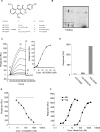
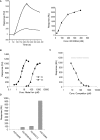
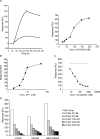
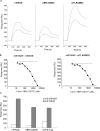
Despite more than 25 years of research, the molecular targets of quinoline-3-carboxamides have been elusive although these compounds are currently in Phase II and III development for treatment of autoimmune/inflammatory diseases in humans. Using photoaffinity cross-linking of a radioactively labelled quinoline-3-carboxamide compound, we could determine a direct association between human S100A9 and quinoline-3-carboxamides. This interaction was strictly dependent on both Zn++ and Ca++. We also show that S100A9 in the presence of Zn++ and Ca++ is an efficient ligand of receptor for advanced glycation end products (RAGE) and also an endogenous Toll ligand in that it shows a highly specific interaction with TLR4/MD2. Both these interactions are inhibited by quinoline-3-carboxamides. A clear structure-activity relationship (SAR) emerged with regard to the binding of quinoline-3-carboxamides to S100A9, as well as these compounds potency to inhibit interactions with RAGE or TLR4/MD2. The same SAR was observed when the compound's ability to inhibit acute experimental autoimmune encephalomyelitis in mice in vivo was analysed. Quinoline-3-carboxamides would also inhibit TNFalpha release in a S100A9-dependent model in vivo, as would antibodies raised against the quinoline-3-carboxamide-binding domain of S100A9. Thus, S100A9 appears to be a focal molecule in the control of autoimmune disease via its interactions with proinflammatory mediators. The specific binding of quinoline-3-carboxamides to S100A9 explains the immunomodulatory activity of this class of compounds and defines S100A9 as a novel target for treatment of human autoimmune diseases.
Conflict of interest statement
Competing interests. PB, AB, MS, DL, AO, and TL are employees of Active Biotech, which is developing quinolines for commercial purposes. FI has a research grant from Active Biotech.
Figures
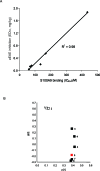

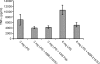
References
-
- Andersen O, Lycke J, Tollesson PO, Svenningsson A, Runmarker B, et al. Linomide reduces the rate of active lesions in relapsing-remitting multiple sclerosis. Neurology. 1996;47:895–900. - PubMed
-
- Karussis DM, Meiner Z, Lehmann D, Gomori JM, Schwarz A, et al. Treatment of secondary progressive multiple sclerosis with the immunomodulator linomide: a double-blind, placebo-controlled pilot study with monthly magnetic resonance imaging evaluation. Neurology. 1996;47:341–346. - PubMed
-
- Noseworthy JH, Wolinsky JS, Lublin FD, Whitaker JN, Linde A, et al. Linomide in relapsing and secondary progressive MS: part I: trial design and clinical results. North American Linomide Investigators. Neurology. 2000;54:1726–1733. - PubMed
-
- Polman C, Barkhof F, Sandberg-Wollheim M, Linde A, Nordle O, et al. Treatment with laquinimod reduces development of active MRI lesions in relapsing MS. Neurology. 2005;64:987–991. - PubMed
-
- Coutant R, Landais P, Rosilio M, Johnsen C, Lahlou N, et al. Low dose linomide in Type I juvenile diabetes of recent onset: a randomised placebo-controlled double blind trial. Diabetologia. 1998;41:1040–1046. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

