Developmental regulation of the intracellular Ca2+ sensitivity of vesicle fusion and Ca2+-secretion coupling at the rat calyx of Held
- PMID: 19403608
- PMCID: PMC2718258
- DOI: 10.1113/jphysiol.2009.172387
Developmental regulation of the intracellular Ca2+ sensitivity of vesicle fusion and Ca2+-secretion coupling at the rat calyx of Held
Abstract
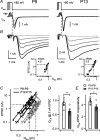
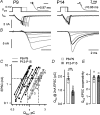
Developmental refinement of synaptic transmission can occur via changes in several pre- and postsynaptic factors, but it has been unknown whether the intrinsic Ca2+ sensitivity of vesicle fusion in the nerve terminal can be regulated during development. Using the calyx of Held, a giant synapse in the auditory pathway, we studied the presynaptic mechanisms underlying the developmental regulation of Ca2+-secretion coupling, comparing a time period before, and shortly after the onset of hearing in rats. We found an approximately 2-fold leftward shift in the relationship between EPSC amplitude and presynaptic Ca2+ current charge (QCa), indicating that brief presynaptic Ca2+ currents become significantly more efficient in driving release. Using a Ca2+ tail current protocol, we also found that the high cooperativity between EPSC amplitude and QCa was slightly reduced with development. In contrast, in presynaptic Ca2+ uncaging experiments, the intrinsic Ca2+ cooperativity of vesicle fusion was identical, and the intrinsic Ca2+ sensitivity was slightly reduced with development. This indicates that the significantly enhanced release efficiency of brief Ca2+ currents must be caused by a tighter co-localization of Ca2+ channels and readily releasable vesicles, but not by changes in the intrinsic properties of Ca2+-dependent release. Using the parameters of the intrinsic Ca2+ sensitivity measured at each developmental stage, we estimate that during a presynaptic action potential (AP), a given readily releasable vesicle experiences an about 1.3-fold higher 'local' intracellular Ca2+ concentration ([Ca2+]i) signal with development. Thus, the data indicate a tightening in the Ca2+ channel-vesicle co-localization during development, without a major change in the intrinsic Ca2+ sensitivity of vesicle fusion.
Figures
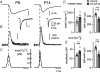
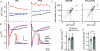

Similar articles
-
Presynaptic capacitance measurements and Ca2+ uncaging reveal submillisecond exocytosis kinetics and characterize the Ca2+ sensitivity of vesicle pool depletion at a fast CNS synapse.J Neurosci. 2003 Aug 6;23(18):7059-68. doi: 10.1523/JNEUROSCI.23-18-07059.2003. J Neurosci. 2003. PMID: 12904466 Free PMC article.
-
A mechanism intrinsic to the vesicle fusion machinery determines fast and slow transmitter release at a large CNS synapse.J Neurosci. 2007 Mar 21;27(12):3198-210. doi: 10.1523/JNEUROSCI.4471-06.2007. J Neurosci. 2007. PMID: 17376981 Free PMC article.
-
cAMP modulates intracellular Ca2+ sensitivity of fast-releasing synaptic vesicles at the calyx of Held synapse.J Neurophysiol. 2010 Dec;104(6):3250-60. doi: 10.1152/jn.00685.2010. Epub 2010 Sep 22. J Neurophysiol. 2010. PMID: 20861434
-
Presynaptic calcium and control of vesicle fusion.Curr Opin Neurobiol. 2005 Jun;15(3):266-74. doi: 10.1016/j.conb.2005.05.006. Curr Opin Neurobiol. 2005. PMID: 15919191 Review.
-
The calyx of Held synapse: from model synapse to auditory relay.Annu Rev Physiol. 2012;74:199-224. doi: 10.1146/annurev-physiol-020911-153236. Epub 2011 Oct 24. Annu Rev Physiol. 2012. PMID: 22035348 Review.
Cited by
-
Presynaptic Rac1 controls synaptic strength through the regulation of synaptic vesicle priming.Elife. 2022 Oct 10;11:e81505. doi: 10.7554/eLife.81505. Elife. 2022. PMID: 36214784 Free PMC article.
-
An Exclusion Zone for Ca2+ Channels around Docked Vesicles Explains Release Control by Multiple Channels at a CNS Synapse.PLoS Comput Biol. 2015 May 7;11(5):e1004253. doi: 10.1371/journal.pcbi.1004253. eCollection 2015 May. PLoS Comput Biol. 2015. PMID: 25951120 Free PMC article.
-
Calcium dependence of neurotransmitter release at a high fidelity synapse.Elife. 2021 Oct 6;10:e70408. doi: 10.7554/eLife.70408. Elife. 2021. PMID: 34612812 Free PMC article.
-
Septins regulate developmental switching from microdomain to nanodomain coupling of Ca(2+) influx to neurotransmitter release at a central synapse.Neuron. 2010 Jul 15;67(1):100-15. doi: 10.1016/j.neuron.2010.06.003. Neuron. 2010. PMID: 20624595 Free PMC article.
-
Molecular mechanisms governing Ca(2+) regulation of evoked and spontaneous release.Nat Neurosci. 2015 Jul;18(7):935-41. doi: 10.1038/nn.4044. Nat Neurosci. 2015. PMID: 26108721 Review.
References
-
- Augustine GJ, Adler EM, Charlton MP. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann N Y Acad Sci. 1991;635:365–381. - PubMed
-
- Bollmann JH, Sakmann B. Control of synaptic strength and timing by the release-site Ca2+ signal. Nat Neurosci. 2005;8:426–434. - PubMed
-
- Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

