Mast cell-cholinergic nerve interaction in mouse airways
- PMID: 19403609
- PMCID: PMC2727042
- DOI: 10.1113/jphysiol.2009.173054
Mast cell-cholinergic nerve interaction in mouse airways
Abstract
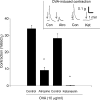
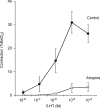
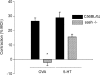
We addressed the mechanism by which antigen contracts trachea isolated from actively sensitized mice. Trachea were isolated from mice (C57BL/6J) that had been actively sensitized to ovalbumin (OVA). OVA (10 microg ml(-1)) caused histamine release (approximately total tissue content), and smooth muscle contraction that was rapid in onset and short-lived (t(1/2) < 1 min), reaching approximately 25% of the maximum tissue response. OVA contraction was mimicked by 5-HT, and responses to both OVA and 5-HT were sensitive to 10 microm-ketanserin (5-HT(2) receptor antagonist) and strongly inhibited by atropine (1microm). Epithelial denudation had no effect on the OVA-induced contraction. Histological assessment revealed about five mast cells/tracheal section the vast majority of which contained 5-HT. There were virtually no mast cells in the mast cell-deficient (sash -/-) mouse trachea. OVA failed to elicit histamine release or contractile responses in trachea isolated from sensitized mast cell-deficient (sash -/-) mice. Intracellular recordings of the membrane potential of parasympathetic neurons in mouse tracheal ganglia revealed a ketanserin-sensitive 5-HT-induced depolarization and similar depolarization in response to OVA challenge. These data support the hypothesis that antigen-induced contraction of mouse trachea is epithelium-independent, and requires mast cell-derived 5-HT to activate 5-HT(2) receptors on parasympathetic cholinergic neurons. This leads to acetylcholine release from nerve terminals, and airway smooth muscle contraction.
Figures



References
-
- Adams GK, 3rd, Lichtenstein L. In vitro studies of antigen-induced bronchospasm: effect of antihistamine and SRS-A antagonist on response of sensitized guinea pig and human airways to antigen. J Immunol. 1979;122:555–562. - PubMed
-
- Aitken MM, Deline TR, Eyre P. Influence of the method of sensitization on some features of anaphylaxis in calves. J Comp Pathol. 1975;85:351–360. - PubMed
-
- Bjorck T, Dahlen SE. Leukotrienes and histamine mediate IgE-dependent contractions of human bronchi: pharmacological evidence obtained with tissues from asthmatic and non-asthmatic subjects. Pulm Pharmacol. 1993;6:87–96. - PubMed
-
- Chand N, Eyre P. Pharmacological study of chicken airway smooth muscle. J Pharm Pharmacol. 1978;30:432–435. - PubMed
-
- Chand N, Eyre P. Reactivity of isolated canine bronchus and pulmonary blood vessels to autonomic, autacoid agents and antigen. Agents Actions. 1979;9:4–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

