Multiple roles for Sox2 in the developing and adult mouse trachea
- PMID: 19403656
- PMCID: PMC2680112
- DOI: 10.1242/dev.034629
Multiple roles for Sox2 in the developing and adult mouse trachea
Abstract
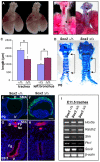
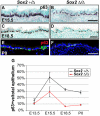
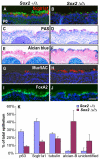
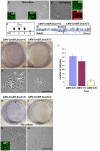
The esophagus, trachea and lung develop from the embryonic foregut, yet acquire and maintain distinct tissue phenotypes. Previously, we demonstrated that the transcription factor Sox2 is necessary for foregut morphogenesis and esophagus development. We show that Sox2 is also required for the normal development of the trachea and lung. In both the embryo and adult, Sox2 is exclusively expressed in the epithelium of the trachea and airways. We use an Nkx2.5-Cre transgene and a Sox2 floxed allele to conditionally delete Sox2 in the ventral epithelial domain of the early anterior foregut, which gives rise to the future trachea and lung buds. All conditional mutants die of respiratory distress at birth, probably due to abnormal differentiation of the laryngeal and tracheal cartilage as a result of defective epithelial-mesenchymal interaction. About 60% of the mutants have a short trachea, suggesting that the primary budding site of the lung shifts anteriorly. In the tracheal epithelium of all conditional mutants there are significantly more mucus-producing cells compared with wild type, and fewer basal stem cells, ciliated and Clara cells. Differentiation of the epithelium lining the conducting airways in the lung is abnormal, suggesting that Sox2 also plays a role in the differentiation of embryonic airway progenitors into specific lineages. Conditional deletion of Sox2 was then used to test its role in adult epithelium maintenance. We found that epithelial cells, including basal stem cells, lacking Sox2 show a reduced capacity to proliferate in culture and to repair after injury in vivo. Taken together, these results define multiple roles for Sox2 in the developing and adult trachea.
Figures



References
-
- Aubin, J., Lemieux, M., Tremblay, M., Berard, J. and Jeannotte, L. (1997). Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev. Biol. 192, 432-445. - PubMed
-
- Cardoso, W. V. and Lu, J. (2006). Regulation of early lung morphogenesis: questions, facts and controversies. Development 133, 1611-1624. - PubMed
-
- Chen, F., Desai, T. J., Qian, J., Niederreither, K., Lu, J. and Cardoso, W. V. (2007). Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 134, 2969-2979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

